当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of Substrate Recognition and Biosynthesis in Class IV Lanthipeptide Systems
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-04-10 , DOI: 10.1021/jacs.8b01323 Julian D Hegemann 1 , Wilfred A van der Donk 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-04-10 , DOI: 10.1021/jacs.8b01323 Julian D Hegemann 1 , Wilfred A van der Donk 1
Affiliation
|
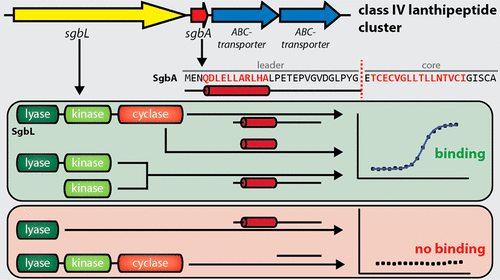
Lanthipeptides belong to the family of ribosomally synthesized and post-translationally modified peptides (RiPPs) and are subdivided into four classes. The first two classes have been heavily studied, but less is known about classes III and IV. The lanthipeptide synthetases of classes III and IV share a similar organization of protein domains: A lyase domain at the N-terminus, a central kinase domain, and a C-terminal cyclase domain. Here, we provide deeper insight into class IV enzymes (LanLs). A series of putative producer strains was screened to identify production conditions of four new venezuelin-like lanthipeptides, and an Escherichia coli based heterologous production system was established for a fifth. The latter not only allowed production of fully modified core peptide but was also employed as the basis for mutational analysis of the precursor peptide to identify regions important for enzyme recognition. These experiments were complemented by in vitro binding studies aimed at identifying the region of the leader peptide recognized by the LanL enzymes as well as determining which domain of the enzyme is recognizing the substrate peptide. Combined, these studies revealed that the kinase domain is mediating the interaction with the precursor peptide and that a putatively α-helical stretch of residues at the center to N-terminal region of the leader peptide is important for enzyme recognition. In addition, a combination of in vitro assays and tandem mass spectrometry was used to elucidate the order of dehydration events in these systems.
中文翻译:

IV 类羊毛硫肽系统中底物识别和生物合成的研究
Lanthipeptides 属于核糖体合成和翻译后修饰肽 (RiPP) 家族,分为四类。前两类已被深入研究,但对 III 类和 IV 类知之甚少。III 类和 IV 类的羊毛硫肽合成酶具有相似的蛋白质结构域组织:N 末端的裂解酶结构域、中央激酶结构域和 C 末端环化酶结构域。在这里,我们对 IV 类酶 (LanLs) 进行了更深入的了解。筛选了一系列假定的生产菌株,以确定四种新的类委内瑞拉羊毛硫肽的生产条件,并建立了第五种基于大肠杆菌的异源生产系统。后者不仅可以生产完全修饰的核心肽,而且还可以用作前体肽突变分析的基础,以识别对酶识别重要的区域。这些实验得到了体外结合研究的补充,旨在鉴定 LanL 酶识别的前导肽区域以及确定酶的哪个结构域正在识别底物肽。综合起来,这些研究表明激酶结构域介导与前体肽的相互作用,并且前导肽中心至 N 末端区域的残基的推定 α 螺旋延伸对于酶识别非常重要。此外,结合体外测定和串联质谱分析来阐明这些系统中脱水事件的顺序。
更新日期:2018-04-10
中文翻译:

IV 类羊毛硫肽系统中底物识别和生物合成的研究
Lanthipeptides 属于核糖体合成和翻译后修饰肽 (RiPP) 家族,分为四类。前两类已被深入研究,但对 III 类和 IV 类知之甚少。III 类和 IV 类的羊毛硫肽合成酶具有相似的蛋白质结构域组织:N 末端的裂解酶结构域、中央激酶结构域和 C 末端环化酶结构域。在这里,我们对 IV 类酶 (LanLs) 进行了更深入的了解。筛选了一系列假定的生产菌株,以确定四种新的类委内瑞拉羊毛硫肽的生产条件,并建立了第五种基于大肠杆菌的异源生产系统。后者不仅可以生产完全修饰的核心肽,而且还可以用作前体肽突变分析的基础,以识别对酶识别重要的区域。这些实验得到了体外结合研究的补充,旨在鉴定 LanL 酶识别的前导肽区域以及确定酶的哪个结构域正在识别底物肽。综合起来,这些研究表明激酶结构域介导与前体肽的相互作用,并且前导肽中心至 N 末端区域的残基的推定 α 螺旋延伸对于酶识别非常重要。此外,结合体外测定和串联质谱分析来阐明这些系统中脱水事件的顺序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号