当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-iron catalysts for electrochemical water oxidation – redox synergism investigated by in situ X-ray spectroscopy with millisecond time resolution†
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8se00114f Diego González-Flores 1, 2, 3, 4, 5 , Katharina Klingan 1, 2, 3, 4 , Petko Chernev 1, 2, 3, 4 , Stefan Loos 1, 2, 3, 4 , Mohammad Reza Mohammadi 1, 2, 3, 4 , Chiara Pasquini 1, 2, 3, 4 , Paul Kubella 1, 2, 3, 4 , Ivelina Zaharieva 1, 2, 3, 4 , Rodney D. L. Smith 1, 2, 3, 4 , Holger Dau 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8se00114f Diego González-Flores 1, 2, 3, 4, 5 , Katharina Klingan 1, 2, 3, 4 , Petko Chernev 1, 2, 3, 4 , Stefan Loos 1, 2, 3, 4 , Mohammad Reza Mohammadi 1, 2, 3, 4 , Chiara Pasquini 1, 2, 3, 4 , Paul Kubella 1, 2, 3, 4 , Ivelina Zaharieva 1, 2, 3, 4 , Rodney D. L. Smith 1, 2, 3, 4 , Holger Dau 1, 2, 3, 4
Affiliation
|
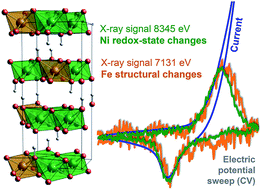
In future technological systems for chemical storage of renewable energy and production of non-fossil fuels, NiFe oxyhydroxides are prime candidates for efficient alkaline water oxidation (oxygen evolution reaction, OER). The synergistic effect of Ni and Fe is well documented but still insufficiently understood. Fluorescence-detected X-ray absorption spectroscopy at the K-edges of Ni and Fe provided structural information on the non-catalytic (reduced) and catalytic (oxidized) state of the NiFe catalyst. Time-resolved detection of X-ray signals during (i) cyclic voltammetry and (ii) in response to potential steps revealed that the Ni(2+)/Ni(3+) redox transition is directly coupled to modification of the Fe ligand environment. We propose that the lattice-geometry modification of the Ni(Fe) oxyhydroxide that results from Ni oxidation enforces changes in the ligand environment of the Fe ions. The Fe sites do not undergo a distinctive redox transition, but are “enslaved” by the oxidation state changes of the Ni ions.
中文翻译:

用于电化学水氧化的镍铁催化剂–通过毫秒时间分辨率的原位X射线光谱研究了氧化还原协同作用†
在用于可再生能源的化学存储和非化石燃料生产的未来技术系统中,羟基氧化镍铁是有效碱性水氧化(放氧反应,OER)的主要候选者。镍和铁的协同作用已得到充分证明,但仍缺乏足够的了解。Ni和Fe的K边缘的荧光检测X射线吸收光谱提供了有关NiFe催化剂的非催化(还原)和催化(氧化)状态的结构信息。在(i)循环伏安法和(ii)响应潜在步骤过程中对X射线信号的时间分辨检测表明,Ni(2 +)/ Ni(3+)氧化还原转变直接与铁配体环境的修饰耦合。我们提出由Ni氧化引起的Ni(Fe)羟基氧化物的晶格几何修饰会强制Fe离子的配体环境发生变化。Fe位点没有经历明显的氧化还原转变,而是被Ni离子的氧化态变化“奴役”。
更新日期:2018-04-10
中文翻译:

用于电化学水氧化的镍铁催化剂–通过毫秒时间分辨率的原位X射线光谱研究了氧化还原协同作用†
在用于可再生能源的化学存储和非化石燃料生产的未来技术系统中,羟基氧化镍铁是有效碱性水氧化(放氧反应,OER)的主要候选者。镍和铁的协同作用已得到充分证明,但仍缺乏足够的了解。Ni和Fe的K边缘的荧光检测X射线吸收光谱提供了有关NiFe催化剂的非催化(还原)和催化(氧化)状态的结构信息。在(i)循环伏安法和(ii)响应潜在步骤过程中对X射线信号的时间分辨检测表明,Ni(2 +)/ Ni(3+)氧化还原转变直接与铁配体环境的修饰耦合。我们提出由Ni氧化引起的Ni(Fe)羟基氧化物的晶格几何修饰会强制Fe离子的配体环境发生变化。Fe位点没有经历明显的氧化还原转变,而是被Ni离子的氧化态变化“奴役”。











































 京公网安备 11010802027423号
京公网安备 11010802027423号