当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Higher Alcohols Synthesis over Carbon Nanohorn-Supported KCoRhMo Catalyst: Pelletization and Kinetic Modeling
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-13 , DOI: 10.1021/acs.iecr.7b03760 Philip E. Boahene 1, 2 , Ajay K. Dalai 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-13 , DOI: 10.1021/acs.iecr.7b03760 Philip E. Boahene 1, 2 , Ajay K. Dalai 1, 2
Affiliation
|
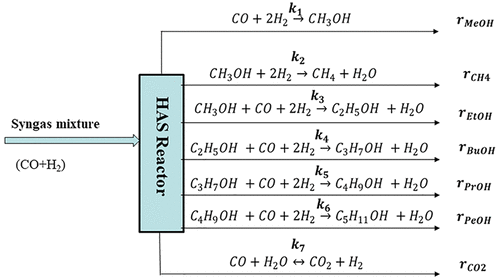
In this study, two catalyst grain sizes (fine powders and pellets) have been investigated to elucidate the effects of both external and internal mass transfer diffusion as well as particle size during the CO hydrogenation reaction to produce higher alcohols. Catalyst grain sizes of 88 and 254 μm were advisedly chosen based on our previous investigations with a similar catalyst matrix for the higher alcohol synthesis (HAS) reaction. The focus in this work was to explore the attractive textural properties of CNH support for CO hydrogenation reaction to produce higher alcohols under specified reaction conditions. Also, to ascertain the possibilities of commercialization of the developed carbon nanohorn (CNH)-supported KCoRhMo catalyst, bentonite clay was added to the pristine support as a binder to enhance its mechanical/crushing strength and kinetic analyses of such catalyst assessed. The influence of bentonite clay was investigated by incorporating 5 wt % of this binder into the formulation of the KCoRhMo/CNH catalyst. For mass transfer considerations, the finely ground KCoRhMo/CNH catalyst powder of 88 μm particle size was used so as to eradicate mass transfer resistance. CO hydrogenation experiments were carried out using a two-phase fixed-bed reactor system to ascertain the intrinsic kinetics of the liquid alcohol products (methanol, ethanol, higher alcohols) as well as the gaseous products generated by the reaction. Syngas of H2/CO ratio of 1.25 was used as feedstock at temperatures, pressures, and gas hourly space velocity ranges of 290–350 °C, 800–1400 psig, and 2.4–4.8 m3 STP/kgcat/h, respectively. The power law model was used to fit experimental data to evaluate kinetic parameters for the HAS reaction on the catalyst surface. Fitting of the experimental data with the developed power law models showed good fits with high R2 values in the range of 0.88–0.96 for the components evaluated. The activation energies computed for ethanol and propanol in the HAS reaction over CNH-supported KCoRhMo catalyst were 54.4 and 92.2 kJ/mol, which are low compared to values obtained by other researchers in similar studies.
中文翻译:

碳纳米角负载的KCoRhMo催化剂上的高级醇合成:造粒和动力学建模
在这项研究中,已研究了两种催化剂粒度(细粉和粒料),以阐明内部和内部传质扩散的影响以及在CO加氢反应生成高级醇的过程中粒径的影响。建议根据我们先前的研究,选择催化剂粒度为88和254μm,并使用类似的催化剂基质进行高级醇合成(HAS)反应。这项工作的重点是探讨CNH载体在指定的反应条件下对CO加氢反应以生产高级醇的有吸引力的质构特性。另外,为了确定开发的碳纳米角(CNH)负载的KCoRhMo催化剂的商业化可能性,将膨润土作为粘合剂加入到原始载体中,以增强其机械/抗碎强度,并评估该催化剂的动力学。通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气在290–350°C,800–1400 psig和2.4–4.8 m 3 STP / kg cat / h的温度,压力和气时空速范围内,将1.25的2 / CO比用作原料。使用幂律模型拟合实验数据,以评估催化剂表面上HAS反应的动力学参数。实验数据与已开发的幂定律模型的拟合表明,对于所评估的组件,R 2值在0.88–0.96的范围内具有良好的拟合度。在CNH负载的KCoRhMo催化剂上,HAS反应中乙醇和丙醇的活化能为54.4和92.2 kJ / mol,与其他研究人员在类似研究中获得的值相比较低。
更新日期:2018-04-13
中文翻译:

碳纳米角负载的KCoRhMo催化剂上的高级醇合成:造粒和动力学建模
在这项研究中,已研究了两种催化剂粒度(细粉和粒料),以阐明内部和内部传质扩散的影响以及在CO加氢反应生成高级醇的过程中粒径的影响。建议根据我们先前的研究,选择催化剂粒度为88和254μm,并使用类似的催化剂基质进行高级醇合成(HAS)反应。这项工作的重点是探讨CNH载体在指定的反应条件下对CO加氢反应以生产高级醇的有吸引力的质构特性。另外,为了确定开发的碳纳米角(CNH)负载的KCoRhMo催化剂的商业化可能性,将膨润土作为粘合剂加入到原始载体中,以增强其机械/抗碎强度,并评估该催化剂的动力学。通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 通过将5重量%的该粘合剂掺入KCoRhMo / CNH催化剂的配方中,研究了膨润土的影响。出于传质的考虑,使用88微米粒径的KCoRhMo / CNH精细研磨催化剂粉末来消除传质阻力。使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气 使用两相固定床反应器系统进行CO加氢实验,以确定液体醇产物(甲醇,乙醇,高级醇)以及反应生成的气态产物的内在动力学。H的合成气在290–350°C,800–1400 psig和2.4–4.8 m 3 STP / kg cat / h的温度,压力和气时空速范围内,将1.25的2 / CO比用作原料。使用幂律模型拟合实验数据,以评估催化剂表面上HAS反应的动力学参数。实验数据与已开发的幂定律模型的拟合表明,对于所评估的组件,R 2值在0.88–0.96的范围内具有良好的拟合度。在CNH负载的KCoRhMo催化剂上,HAS反应中乙醇和丙醇的活化能为54.4和92.2 kJ / mol,与其他研究人员在类似研究中获得的值相比较低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号