当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flavin Storage and Sequestration by Mycobacterium tuberculosis Dodecin
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acsinfecdis.7b00237 Florian Bourdeaux 1 , Christopher A. Hammer 2 , Stephan Vogt 3 , Felix Schweighöfer 2 , Gilbert Nöll 3 , Josef Wachtveitl 2 , Martin Grininger 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acsinfecdis.7b00237 Florian Bourdeaux 1 , Christopher A. Hammer 2 , Stephan Vogt 3 , Felix Schweighöfer 2 , Gilbert Nöll 3 , Josef Wachtveitl 2 , Martin Grininger 1
Affiliation
|
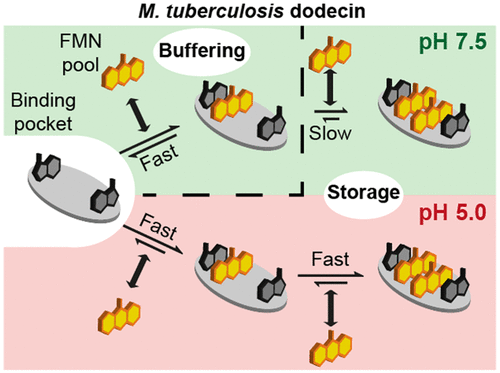
Dodecins are small flavin binding proteins occurring in archaea and bacteria. They are remarkable for binding dimers of flavins with their functional relevant aromatic isoalloxazine rings deeply covered. Bacterial dodecins are widely spread and found in a large variety of pathogens, among them Pseudomonas aeruginosa, Streptococcus pneumonia, Ralstonia solanacearum, and Mycobacterium tuberculosis (M. tuberculosis). In this work, we seek to understand the function of dodecins from M. tuberculosis dodecin. We describe flavin binding in thermodynamic and kinetic properties and achieve mechanistic insight in dodecin function by applying spectroscopic and electrochemical methods. Intriguingly, we reveal a significant pH dependence in the affinity and specificity of flavin binding. Our data give insight in M. tuberculosis dodecin function and advance the current understanding of dodecins as flavin storage and sequestering proteins. We suggest that the dodecin in M. tuberculosis may specifically be important for flavin homeostasis during the elaborate lifestyle of this organism, which calls for the evaluation of this protein as drug target.
中文翻译:

黄素的存储和结核分枝杆菌十二烷的螯合
Dodecins是存在于古细菌和细菌中的小黄素结合蛋白。它们对于将黄素的二聚体与功能相关的芳香异恶嗪环深覆盖在一起具有显着的作用。细菌十二指肠广泛分布于多种病原体中,其中包括铜绿假单胞菌,肺炎链球菌,茄形青枯菌和结核分枝杆菌(M. tuberculosis)。在这项工作中,我们试图了解结核分枝杆菌的十二烷的功能dodecin。我们描述了黄素结合的热力学和动力学特性,并通过应用光谱学和电化学方法获得了十二烷功能的机械学见解。有趣的是,我们揭示了黄素结合的亲和力和特异性对pH的依赖性很大。我们的数据可深入了解结核分枝杆菌的十二指肠功能,并促进当前对十二烷作为黄素贮藏和螯合蛋白的理解。我们建议在结核分枝杆菌中,十二指肠素对于黄素稳态在该生物体精心打造的生活方式中可能特别重要,这要求将该蛋白作为药物靶标进行评估。
更新日期:2018-04-02
中文翻译:

黄素的存储和结核分枝杆菌十二烷的螯合
Dodecins是存在于古细菌和细菌中的小黄素结合蛋白。它们对于将黄素的二聚体与功能相关的芳香异恶嗪环深覆盖在一起具有显着的作用。细菌十二指肠广泛分布于多种病原体中,其中包括铜绿假单胞菌,肺炎链球菌,茄形青枯菌和结核分枝杆菌(M. tuberculosis)。在这项工作中,我们试图了解结核分枝杆菌的十二烷的功能dodecin。我们描述了黄素结合的热力学和动力学特性,并通过应用光谱学和电化学方法获得了十二烷功能的机械学见解。有趣的是,我们揭示了黄素结合的亲和力和特异性对pH的依赖性很大。我们的数据可深入了解结核分枝杆菌的十二指肠功能,并促进当前对十二烷作为黄素贮藏和螯合蛋白的理解。我们建议在结核分枝杆菌中,十二指肠素对于黄素稳态在该生物体精心打造的生活方式中可能特别重要,这要求将该蛋白作为药物靶标进行评估。









































 京公网安备 11010802027423号
京公网安备 11010802027423号