当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Positron Emission Tomography Tau Tracers Bind to Multiple Binding Sites on the Tau Fibril: Insight from Computational Modeling
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acschemneuro.8b00093 N. Arul Murugan 1 , Agneta Nordberg 2, 3 , Hans Ågren 1, 4
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1021/acschemneuro.8b00093 N. Arul Murugan 1 , Agneta Nordberg 2, 3 , Hans Ågren 1, 4
Affiliation
|
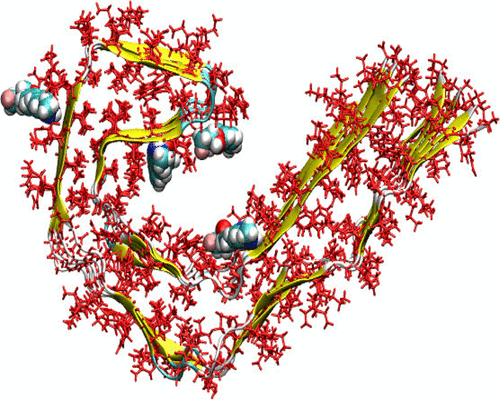
Using the recently reported cryo-EM structure for the tau fibril [Fitzpatrick et al. (2017) Nature 547, 185–190], which is a potential target concerning Alzheimer’s disease, we present the first molecular modeling studies on its interaction with various positron emission tomography (PET) tracers. Experimentally, based on the binding assay studies, at least three different high-affinity binding sites have been reported for tracers in the tau fibril. Herein, through integrated modeling using molecular docking, molecular dynamics, and binding free energy calculations, we provide insight into the binding patterns of various tracers to the tau fibril. We suggest that there are four different high-affinity binding sites available for many of the studied tracers showing varying binding affinity to different binding sites. Thus, PBB3 binds most strongly to site 4, and interestingly, this site is not a preferable site for any other tracers. For THK5351, our data show that it strongly binds to sites 3 and 1, the former one being more preferable. We also find that MK6240 and T807 bind to site 1 specifically. The modeling data also give some insight into whether a tracer bound to a specific site can be replaced by others or not. For example, the displacement of T807 by PBB3 as reported experimentally can also be explained and attributed to the larger binding affinity of the latter compound in all binding sites. The binding free energy results explain very well the small binding affinity of THK523 compared to all the aryl quinoline moieties containing THK tracers. The ability of certain tau tracers, like FDDNP and THK523, to bind to amyloid fibrils has also been investigated. Furthermore, such off-target interaction of tau tracers with amyloid beta fibrils has been validated using a quantum mechanical fragmentation approach.
中文翻译:

不同的正电子发射断层扫描Tau示踪剂与Tau原纤维上的多个结合位点结合:计算建模的见解
使用最近报道的tau原纤维的cryo-EM结构[Fitzpatrick等。(2017)自然547,185–190],这是有关阿尔茨海默氏病的潜在靶标,我们提出了有关其与各种正电子发射断层扫描(PET)示踪剂相互作用的第一个分子模型研究。在实验上,基于结合试验研究,已经报道了tau原纤维中示踪剂的至少三个不同的高亲和力结合位点。在本文中,通过使用分子对接,分子动力学和结合自由能计算的集成模型,我们可以洞察各种示踪剂与tau原纤维的结合模式。我们建议对于许多研究示踪剂有四个不同的高亲和力结合位点,它们显示出对不同结合位点的不同结合亲和力。因此,PBB3与位点4的结合最牢固,有趣的是,该位点不是任何其他示踪剂的优选位点。对于THK5351,我们的数据显示它与3号和1号位点牢固结合,前者更可取。我们还发现MK6240和T807特异性结合位点1。建模数据还使您可以了解绑定到特定站点的示踪剂是否可以被其他站点替换。例如,也可以解释实验报道的T807被PBB3取代,并归因于后一种化合物在所有结合位点中具有更大的结合亲和力。与所有含有THK示踪剂的芳基喹啉部分相比,结合自由能的结果很好地解释了THK523的小的结合亲和力。还研究了某些tau示踪剂(如FDDNP和THK523)与淀粉样蛋白原纤维结合的能力。此外,
更新日期:2018-04-09
中文翻译:

不同的正电子发射断层扫描Tau示踪剂与Tau原纤维上的多个结合位点结合:计算建模的见解
使用最近报道的tau原纤维的cryo-EM结构[Fitzpatrick等。(2017)自然547,185–190],这是有关阿尔茨海默氏病的潜在靶标,我们提出了有关其与各种正电子发射断层扫描(PET)示踪剂相互作用的第一个分子模型研究。在实验上,基于结合试验研究,已经报道了tau原纤维中示踪剂的至少三个不同的高亲和力结合位点。在本文中,通过使用分子对接,分子动力学和结合自由能计算的集成模型,我们可以洞察各种示踪剂与tau原纤维的结合模式。我们建议对于许多研究示踪剂有四个不同的高亲和力结合位点,它们显示出对不同结合位点的不同结合亲和力。因此,PBB3与位点4的结合最牢固,有趣的是,该位点不是任何其他示踪剂的优选位点。对于THK5351,我们的数据显示它与3号和1号位点牢固结合,前者更可取。我们还发现MK6240和T807特异性结合位点1。建模数据还使您可以了解绑定到特定站点的示踪剂是否可以被其他站点替换。例如,也可以解释实验报道的T807被PBB3取代,并归因于后一种化合物在所有结合位点中具有更大的结合亲和力。与所有含有THK示踪剂的芳基喹啉部分相比,结合自由能的结果很好地解释了THK523的小的结合亲和力。还研究了某些tau示踪剂(如FDDNP和THK523)与淀粉样蛋白原纤维结合的能力。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号