当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing light hydrocarbon storage and separation through introducing Lewis basic nitrogen sites within a carboxylate-decorated copper–organic framework†
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1039/c8qm00105g Xiuping Liu 1, 2, 3, 4 , Weidong Fan 1, 2, 3, 4 , Minghui Zhang 1, 2, 3, 4 , Guixia Li 1, 2, 3, 4 , Haijun Liu 1, 2, 3, 4 , Daofeng Sun 1, 2, 3, 4 , Lianming Zhao 1, 2, 3, 4 , Houyu Zhu 1, 2, 3, 4 , Wenyue Guo 1, 2, 3, 4
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2018-04-09 00:00:00 , DOI: 10.1039/c8qm00105g Xiuping Liu 1, 2, 3, 4 , Weidong Fan 1, 2, 3, 4 , Minghui Zhang 1, 2, 3, 4 , Guixia Li 1, 2, 3, 4 , Haijun Liu 1, 2, 3, 4 , Daofeng Sun 1, 2, 3, 4 , Lianming Zhao 1, 2, 3, 4 , Houyu Zhu 1, 2, 3, 4 , Wenyue Guo 1, 2, 3, 4
Affiliation
|
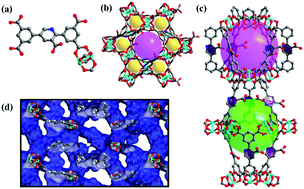
A novel nanoporous Cu metal–organic framework (NEM-4) with open CuII sites, Lewis basic nitrogen sites, and uncoordinated –COO− groups exhibits both outstanding uptake capacities (in cm3 (STP) g−1) for C2H2 (204), C2H4 (164.1), C2H6 (172.2), C3H6 (197.4), and C3H8 (196.1) and high selectivities for C2H2/CH4 (63.2), C3H6/CH4 (174.8), and C3H8/CH4 (168.3) under ambient conditions. After eight cycles of adsorption–desorption tests, only 8.2% and 10.3% decrease in the acetylene and propene storage capacities was observed, indicating an excellent repeatability. Compared with 1 (carboxylate decorated NOTT-101), when nitrogen sites are inserted, the C2–C3 hydrocarbon uptakes of NEM-4 can be significantly enhanced. Grand Canonical Monte Carlo and first-principles calculations reveal that not only the open CuII sites but also the uncoordinated –COO− groups and the nitrogen sites play significant roles in its high C2–C3 hydrocarbon uptakes. Moreover, the adsorption and separation of cationic dyes in NEM-4 are highly size and charge state dependent, and the adsorbed methylene blue (MB+) in NEM-4 can be efficiently released in an NaCl-containing CH3OH solution. This study reveals that the combination of open metal sites, carboxylate groups, Lewis basic pyridyl sites, and appropriate pore geometry is responsible for the high adsorption/separation of light hydrocarbons in NEM-4.
中文翻译:

通过羧酸装饰铜-有机骨架内引入路易斯碱性氮位点提高光碳氢化合物储存和分离†
一种新颖的纳米多孔铜金属-有机骨架(NEM-4)与打开的Cu II位点,路易斯碱性氮位点,和不协调的-COO -基团表现出杰出的两个吸附容量(以cm 3(STP)克-1)对C 2 ħ 2(204),C 2 H 4(164.1),C 2 H 6(172.2),C 3 H 6(197.4)和C 3 H 8(196.1)以及对C 2 H 2 / CH 4(63.2 )的高选择性),C 3 H 6 / CH 4(174.8)和C 3 H 8 / CH 4(168.3)在环境条件下。经过八个吸附-解吸测试循环后,仅发现乙炔和丙烯的存储容量分别下降了8.2%和10.3%,这表明其重复性极好。与1(用羧酸盐修饰的NOTT-101)相比,当插入氮位时,NEM-4的C 2 -C 3烃吸收量可以显着提高。蒙特卡罗和第一原理计算表明,不仅是开放的铜II网站也是不协调的-COO -团体和氮的点位在高C发挥作用显著2 -C 3碳氢化合物的吸收。此外,阳离子染料在NEM-4中的吸附和分离高度依赖于大小和电荷状态,并且可以在含NaCl的CH 3 OH溶液中有效释放NEM-4中吸附的亚甲基蓝(MB +)。这项研究表明,开放金属位点,羧酸根基团,路易斯碱性吡啶基位点和适当的孔几何形状的结合是NEM-4中轻烃高吸附/分离的原因。
更新日期:2018-04-09
中文翻译:

通过羧酸装饰铜-有机骨架内引入路易斯碱性氮位点提高光碳氢化合物储存和分离†
一种新颖的纳米多孔铜金属-有机骨架(NEM-4)与打开的Cu II位点,路易斯碱性氮位点,和不协调的-COO -基团表现出杰出的两个吸附容量(以cm 3(STP)克-1)对C 2 ħ 2(204),C 2 H 4(164.1),C 2 H 6(172.2),C 3 H 6(197.4)和C 3 H 8(196.1)以及对C 2 H 2 / CH 4(63.2 )的高选择性),C 3 H 6 / CH 4(174.8)和C 3 H 8 / CH 4(168.3)在环境条件下。经过八个吸附-解吸测试循环后,仅发现乙炔和丙烯的存储容量分别下降了8.2%和10.3%,这表明其重复性极好。与1(用羧酸盐修饰的NOTT-101)相比,当插入氮位时,NEM-4的C 2 -C 3烃吸收量可以显着提高。蒙特卡罗和第一原理计算表明,不仅是开放的铜II网站也是不协调的-COO -团体和氮的点位在高C发挥作用显著2 -C 3碳氢化合物的吸收。此外,阳离子染料在NEM-4中的吸附和分离高度依赖于大小和电荷状态,并且可以在含NaCl的CH 3 OH溶液中有效释放NEM-4中吸附的亚甲基蓝(MB +)。这项研究表明,开放金属位点,羧酸根基团,路易斯碱性吡啶基位点和适当的孔几何形状的结合是NEM-4中轻烃高吸附/分离的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号