当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (±)-Cephanolides B and C via a Palladium-Catalyzed Cascade Cyclization and Late-stage sp3 C–H Bond Oxidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-09 , DOI: 10.1021/jacs.8b03015 Lun Xu 1 , Chao Wang 1 , Ziwei Gao 1 , Yu-Ming Zhao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-09 , DOI: 10.1021/jacs.8b03015 Lun Xu 1 , Chao Wang 1 , Ziwei Gao 1 , Yu-Ming Zhao 1
Affiliation
|
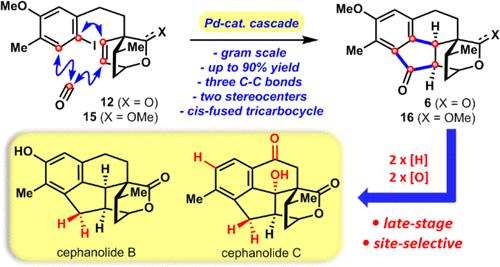
Herein, we report the first total syntheses of complex cephalotaxus diterpenoids cephanolide B and C from commercially available 5-bromo-2-methylanisole. Key to the success of this synthetic route is a palladium-catalyzed cascade cyclization reaction, which allowed us to efficiently forge the 6-5-6 cis-fused tricyclic ring systems found in the entire family of cephalotaxus diterpenoids. Additionally, site-selective late-stage sp3 C-H bond oxidation served as a key strategic element in the chemical synthesis of cephanolide C.
中文翻译:

(±)-头孢内酯 B 和 C 通过钯催化的级联环化和后期 sp3 C-H 键氧化的全合成
在此,我们报告了从市售的 5-溴-2-甲基苯甲醚中首次全合成复杂头杉二萜类头孢内酯 B 和 C。该合成路线成功的关键是钯催化的级联环化反应,这使我们能够有效地构建在整个头杉二萜类化合物中发现的 6-5-6 顺式稠合三环系统。此外,位点选择性后期 sp3 CH 键氧化是头孢内酯 C 化学合成中的关键战略要素。
更新日期:2018-04-09
中文翻译:

(±)-头孢内酯 B 和 C 通过钯催化的级联环化和后期 sp3 C-H 键氧化的全合成
在此,我们报告了从市售的 5-溴-2-甲基苯甲醚中首次全合成复杂头杉二萜类头孢内酯 B 和 C。该合成路线成功的关键是钯催化的级联环化反应,这使我们能够有效地构建在整个头杉二萜类化合物中发现的 6-5-6 顺式稠合三环系统。此外,位点选择性后期 sp3 CH 键氧化是头孢内酯 C 化学合成中的关键战略要素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号