当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Diazo Ketones in On-Surface Chemistry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-08 , DOI: 10.1021/jacs.8b02599 Lacheng Liu 1, 2 , Henning Klaasen , Alexander Timmer 1, 2 , Hong-Ying Gao 1, 2 , Dennis Barton 3 , Harry Mönig 1, 2 , Johannes Neugebauer , Harald Fuchs 1, 2 , Armido Studer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-08 , DOI: 10.1021/jacs.8b02599 Lacheng Liu 1, 2 , Henning Klaasen , Alexander Timmer 1, 2 , Hong-Ying Gao 1, 2 , Dennis Barton 3 , Harry Mönig 1, 2 , Johannes Neugebauer , Harald Fuchs 1, 2 , Armido Studer
Affiliation
|
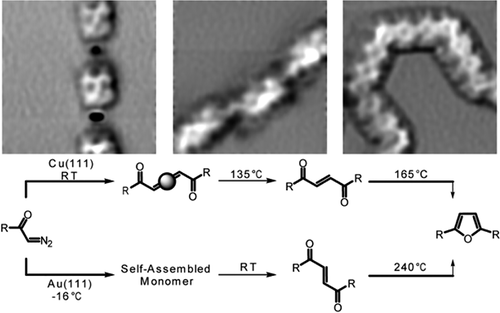
Polymerization of a biphenyl bis α-diazo ketone on Cu(111) and Au(111) surfaces to provide furandiyl bridged poly-para-phenylenes is reported. Polymerization on Cu(111) occurs via initial N2 fragmentation leading to Cu-biscarbene complexes at room temperature as polymeric organometallic structure. At 135 °C, carbene coupling affords polymeric α,β-unsaturated 1,4-diketones, while analogous alkene formation on the Au(111) surface occurs at room temperature. Further temperature increase leads to deoxygenative cyclization of the 1,4-diketone moieties to provide alternating furandiyl biphenyl copolymers on Cu(111) (165 °C) and Au(111) (240 °C) surfaces. This work shows a new approach to generate Cu-biscarbene intermediates on surfaces, opening the pathway for the controlled generation of biphenyl copolymers.
中文翻译:

表面化学中的α-重氮酮
据报道,联苯双 α-重氮酮在 Cu(111) 和 Au(111) 表面聚合以提供呋喃二基桥联聚对亚苯基。Cu(111) 上的聚合通过初始 N2 碎片发生,导致在室温下作为聚合有机金属结构的 Cu-双碳烯配合物。在 135 °C 时,卡宾偶联产生聚合的 α,β-不饱和 1,4-二酮,而在室温下在 Au(111) 表面上形成类似的烯烃。进一步的温度升高导致 1,4-二酮部分的脱氧环化,从而在 Cu(111) (165 °C) 和 Au(111) (240 °C) 表面上提供交替的呋喃二联苯共聚物。这项工作展示了一种在表面生成 Cu-双碳烯中间体的新方法,为控制生成联苯共聚物开辟了道路。
更新日期:2018-04-08
中文翻译:

表面化学中的α-重氮酮
据报道,联苯双 α-重氮酮在 Cu(111) 和 Au(111) 表面聚合以提供呋喃二基桥联聚对亚苯基。Cu(111) 上的聚合通过初始 N2 碎片发生,导致在室温下作为聚合有机金属结构的 Cu-双碳烯配合物。在 135 °C 时,卡宾偶联产生聚合的 α,β-不饱和 1,4-二酮,而在室温下在 Au(111) 表面上形成类似的烯烃。进一步的温度升高导致 1,4-二酮部分的脱氧环化,从而在 Cu(111) (165 °C) 和 Au(111) (240 °C) 表面上提供交替的呋喃二联苯共聚物。这项工作展示了一种在表面生成 Cu-双碳烯中间体的新方法,为控制生成联苯共聚物开辟了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号