Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-04-07 , DOI: 10.1016/j.bmcl.2018.04.003 Munia F Sowaileh 1 , Amy E Salyer 2 , Kuldeep K Roy 1 , Jinu P John 2 , James R Woods 2 , Robert J Doerksen 1 , Gregory H Hockerman 2 , David A Colby 1
|
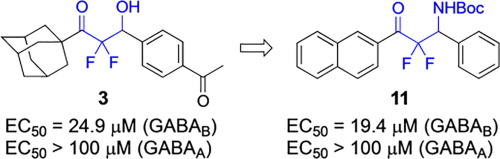
β-Hydroxy difluoromethyl ketones represent the newest class of agonists of the GABA-B receptor, and they are structurally distinct from all other known agonists at this receptor because they do not display the carboxylic acid or amino group of γ-aminobutyric acid (GABA). In this report, the design, synthesis, and biological evaluation of additional analogues of β-hydroxy difluoromethyl ketones characterized the critical nature of the substituted aromatic group on the lead compound. The importance of these new data is interpreted by docking studies using the X-ray structure of the GABA-B receptor. Moreover, we also report that the synthesis and biological evaluation of β-amino difluoromethyl ketones provided the most potent compound across these two series.
中文翻译:

源自 β-羟基和 β-氨基二氟甲基酮的 γ-氨基丁酸 B 型 (GABAB) 受体激动剂
β-羟基二氟甲基酮代表 GABA-B 受体的最新一类激动剂,它们在结构上与该受体的所有其他已知激动剂不同,因为它们不显示 γ-氨基丁酸 (GABA) 的羧酸或氨基。在本报告中,β-羟基二氟甲基酮的其他类似物的设计、合成和生物学评价表征了先导化合物上取代的芳族基团的关键性质。这些新数据的重要性通过使用 GABA-B 受体的 X 射线结构的对接研究来解释。此外,我们还报告称,β-氨基二氟甲基酮的合成和生物学评价提供了这两个系列中最有效的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号