Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2018-03-31 , DOI: 10.1016/j.jssc.2018.03.035 Juan-zhi Yan , Li-ping Lu , Miao-li Zhu , Si-si Feng
|
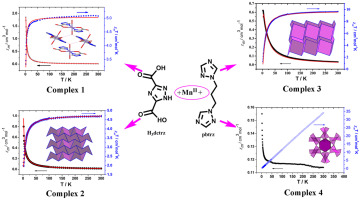
Four manganese (II) compounds are obtained by the reaction of manganese salts, triazole-derivatives and auxiliary reagents in aqueous solution or mix-solvents by routine or hydrothermal reactions. X-ray crystal structure analyses reveal that a neutral 0D compound [Mn(Hmctrz)2(H2O)2] (1) (H2mctrz = 1H-1,2,4-triazole-3-carboxylic acid) displays a centro-symmetric mononuclear octahedral entity with two Hmctrz− anions and two water molecules; two neutral 2D clusters [Mn(Hdctrz)(H2O)2]n (2) (H3dctrz = 1H-1,2,4-triazole-3,5-dicarboxylic acid) and [Mn2(pbtrz)(btca)]n·4nH2O (3) (pbtrz = 1,3-bis(1,2,4-triazol-1-yl)-propane&H4btca = benzene-1,2,4,5-tetracarboxylic acid) possess layer structures with Hdctrz2− linkers (2) and Mn(II)-pbtrz-Mn(II) building blocks periodically extended by μ-btca4− connectors (3); [Mn(pbtrz)]n·nOAc·nOH (4) shows a 3D diamond-shaped cationic framework with the anion void volume of 49.2%. Nitrogenous bases are used as the auxiliary ligand in compound 3 and the temple ligand in compounds 1, 2, and 4. Compounds 1–4 show antiferromagnetic coupling that has been fitted by different models with the molecular field approximate with D = − 0.129(1) cm−1 for 1, J = − 0.354(4) cm−1 for 2 and J = − 0.696(6) cm−1 for 3, respectively. The magnetic differences can be related to different superexchange interactions transmitted by the crystal lattice and/or the zero field splitting (ZFS) of the 6A1g single-ion states of 1 and the syn-anti-COO− of 2 as well as the mixed magnetic bridges of μ1-O and μ-pbtrz-μ-COO− of 3.
中文翻译:

基于双官能团配体的新型锰(II)化合物的自组装:合成,结构和磁性
通过使锰盐,三唑衍生物和辅助试剂在水溶液或混合溶剂中通过常规或水热反应进行反应,可获得四种锰(II)化合物。X射线晶体结构分析显示中性0D化合物[Mn(Hmctrz)2(H 2 O)2 ](1)(H 2 mctrz = 1 H -1,2,4-三唑-3-羧酸)显示一个中心对称的单核八面体实体有两个Hmctrz -阴离子和两个水分子; 两个中性二维簇[Mn(Hdctrz)(H 2 O)2 ] n(2)(H 3 dctrz = 1 H-1,2,4-三唑-3,5-二羧酸)和[Mn 2(pbtrz)(btca)] n ·4nH 2 O(3)(pbtrz = 1,3-双(1,2,4-三唑-1-基) -丙烷&H 4 BTCA =苯-1,2,4,5-四羧酸)与具有层Hdctrz结构2 -的连接体(2)和Mn(II)-pbtrz锰(II)积木周期性通过μ- btca 4 −连接器(3)扩展; [Mn(pbtrz)] n ·nOAc·nOH(4)显示3D菱形阳离子框架,其阴离子空隙体积为49.2%。氮碱用作化合物3中的辅助配体和镜腿化合物中配体1,2,和4。化合物1 - 4显示了已装配由不同的模型与分子场近似与反铁磁耦合d = - 0.129(1)厘米-1为1,Ĵ = - 0.354(4)厘米-1为2和Ĵ = - 0.696 (6)厘米-1为3,分别。磁差可能与6 A 1g的晶格和/或零场分裂(ZFS)传输的不同超交换相互作用有关的单离子状态1和SYN-抗-COO -的2以及的混合磁桥μ 1 -O和μ -pbtrz- μ -COO -的3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号