当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd@TiO2/carbon nanohorn electrocatalysts: reversible CO2 hydrogenation to formic acid†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1039/c7ee03361c M. Melchionna 1, 2, 3, 4, 5 , M. V. Bracamonte 1, 2, 3, 4, 5 , A. Giuliani 1, 2, 3, 4, 5 , L. Nasi 5, 6, 7, 8 , T. Montini 1, 2, 3, 4, 5 , C. Tavagnacco 1, 2, 3, 4, 5 , M. Bonchio 5, 9, 10, 11 , P. Fornasiero 1, 2, 3, 4, 5 , M. Prato 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1039/c7ee03361c M. Melchionna 1, 2, 3, 4, 5 , M. V. Bracamonte 1, 2, 3, 4, 5 , A. Giuliani 1, 2, 3, 4, 5 , L. Nasi 5, 6, 7, 8 , T. Montini 1, 2, 3, 4, 5 , C. Tavagnacco 1, 2, 3, 4, 5 , M. Bonchio 5, 9, 10, 11 , P. Fornasiero 1, 2, 3, 4, 5 , M. Prato 1, 2, 3, 4, 5
Affiliation
|
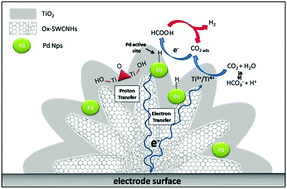
Direct conversion of carbon dioxide to formic acid at thermodynamic equilibrium is an advantage of enzymatic catalysis, hardly replicated by synthetic analogs, but of high priority for carbon-neutral energy schemes. The bio-mimetic potential of totally inorganic Pd@TiO2 nanoparticles is envisioned herein in combination with Single Walled Carbon NanoHorns (SWCNHs). The high surface nano-carbon entanglement templates a wide distribution of “hard-soft” bimetallic sites where the small Pd nanoparticles (1.5 nm) are shielded within the TiO2 phase (Pd@TiO2), while being electrically wired to the electrode by the nanocarbon support. This hybrid electrocatalyst activates CO2 reduction to formic acid at near zero overpotential in the aqueous phase (onset potential at E < −0.05 V vs. RHE, pH = 7.4), while being able to evolve hydrogen via sequential formic acid dehydrogenation. The net result hints at a unique CO2 “circular catalysis” where formic acid versus H2 selectivity is addressable by flow-reactor technology.
中文翻译:

Pd @ TiO 2 /碳纳米角电催化剂:可逆的CO 2加氢成甲酸†
在热力学平衡条件下将二氧化碳直接转化为甲酸是酶催化的优势,几乎不能被合成类似物复制,但是对于碳中和能源计划具有很高的优先级。本文设想了与单壁碳纳米角(SWCNHs)组合的完全无机的Pd @ TiO 2纳米颗粒的仿生潜力。高表面纳米碳缠结模板分布着广泛的“硬-软”双金属位点,其中小的Pd纳米颗粒(1.5 nm)在TiO 2相(Pd @ TiO 2)内被屏蔽,同时通过纳米碳载体。这种杂化电催化剂可活化CO 2在水相中接近零的超电势下还原成甲酸(E <-0.05 V相对于RHE的起始电势,pH = 7.4),同时能够通过顺序的甲酸脱氢生成氢。最终结果暗示了独特的CO 2 “循环催化”,其中甲酸对H 2的选择性可以通过流动反应器技术解决。
更新日期:2018-04-06
中文翻译:

Pd @ TiO 2 /碳纳米角电催化剂:可逆的CO 2加氢成甲酸†
在热力学平衡条件下将二氧化碳直接转化为甲酸是酶催化的优势,几乎不能被合成类似物复制,但是对于碳中和能源计划具有很高的优先级。本文设想了与单壁碳纳米角(SWCNHs)组合的完全无机的Pd @ TiO 2纳米颗粒的仿生潜力。高表面纳米碳缠结模板分布着广泛的“硬-软”双金属位点,其中小的Pd纳米颗粒(1.5 nm)在TiO 2相(Pd @ TiO 2)内被屏蔽,同时通过纳米碳载体。这种杂化电催化剂可活化CO 2在水相中接近零的超电势下还原成甲酸(E <-0.05 V相对于RHE的起始电势,pH = 7.4),同时能够通过顺序的甲酸脱氢生成氢。最终结果暗示了独特的CO 2 “循环催化”,其中甲酸对H 2的选择性可以通过流动反应器技术解决。



























 京公网安备 11010802027423号
京公网安备 11010802027423号