当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature Dependent Rate Coefficients for the Gas-Phase Reaction of the OH Radical with Linear (L2, L3) and Cyclic (D3, D4) Permethylsiloxanes
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1021/acs.jpca.8b01908 François Bernard 1, 2 , Dimitrios K. Papanastasiou 1, 2 , Vassileios C. Papadimitriou 1, 2 , James B. Burkholder 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1021/acs.jpca.8b01908 François Bernard 1, 2 , Dimitrios K. Papanastasiou 1, 2 , Vassileios C. Papadimitriou 1, 2 , James B. Burkholder 1
Affiliation
|
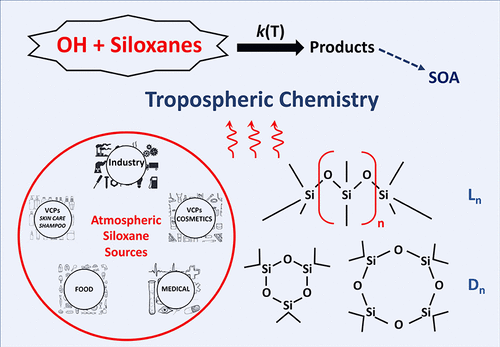
Permethylsiloxanes are emitted into the atmosphere during production and use as personal care products, lubricants, and cleaning agents. The predominate atmospheric loss process for permethylsiloxanes is expected to be via gas-phase reaction with the OH radical. In this study, rate coefficients, k(T), for the OH radical gas-phase reaction with the two simplest linear and cyclic permethylsiloxanes were measured using a pulsed laser photolysis–laser induced fluorescence technique over the temperature range of 240–370 K and a relative rate method at 294 K: hexamethyldisiloxane ((CH3)3SiOSi(CH3)3, L2), k1; octamethyltrisiloxane ([(CH3)3SiO]2Si(CH3)2, L3), k2; hexamethylcyclotrisiloxane ([-Si(CH3)2O-]3, D3), k3; and octamethylcyclotetrasiloxane ([-Si(CH3)2O-]4, D4), k4. The obtained k(294 K) values and temperature-dependence expressions for the 240–370 K temperature range are (cm3 molecule–1 s–1, 2σ absolute uncertainties): k1(294 K) = (1.28 ± 0.08) × 10–12, k1(T) = (1.87 ± 0.18) × 10–11 exp(−(791 ± 27)/T); k2(294 K) = (1.72 ± 0.10) × 10–12, k2(T) = 1.96 × 10–13 (T/298)4.34 exp(657/T); k3(294 K) = (0.82 ± 0.05) × 10–12, k3(T) = (1.29 ± 0.19) × 10–11 exp(−(805 ± 43)/T); and k4(294 K) = (1.12 ± 0.10) × 10–12, k4(T) = (1.80 ± 0.26) × 10–11 exp(−(816 ± 43)/T). The cyclic molecules were found to be less reactive than the analogous linear molecule with the same number of −CH3 groups, while the linear and cyclic permethylsiloxane reactivity both increase with the increasing number of CH3– groups. The present results are compared with previous rate coefficient determinations where available. The permethylsiloxanes included in this study are atmospherically short-lived compounds with estimated atmospheric lifetimes of 11, 8, 17, and 13 days, respectively.
中文翻译:

OH自由基与线性(L 2,L 3)和环状(D 3,D 4)全甲基硅氧烷的气相反应的温度相关速率系数
在生产过程中,全甲基硅氧烷会排放到大气中,并用作个人护理产品,润滑剂和清洁剂。预期全甲基硅氧烷的主要大气损失过程是通过与OH自由基的气相反应。在这项研究中,使用脉冲激光光解-激光诱导荧光技术在240-370 K和温度范围内,用两种最简单的线性和环状全甲基硅氧烷对OH自由基气相反应的速率系数k(T)进行了测量。在294K下的相对速率法:六甲基二硅氧烷((CH 3)3 SiOSi(CH 3)3,L 2),k 1;八甲基三硅氧烷([(CH 3)3 SiO] 2 Si(CH 3)2,L 3),k 2;六甲基环三硅氧烷([-Si(CH 3)2 O-] 3,D 3),k 3;和八甲基环四硅氧烷([-Si(CH 3)2 O-] 4,D 4),k 4。对于240–370 K的温度范围,获得的k(294 K)值和温度依赖性表达式为(cm 3分子–1 s –1,2σ绝对不确定性):k 1(294 K)=(1.28±0.08)×10 –12,k 1(T)=(1.87±0.18)×10 –11 exp(-(791±27)/ T); k 2(294 K)=(1.72±0.10)×10 –12,k 2(T)= 1.96×10 –13(T / 298)4.34 exp(657 / T); k 3(294 K)=(0.82±0.05)×10 –12,k 3(T)=(1.29±0.19)×10 –11 exp(-(805±43)/ T); 和ķ 4(294 K)=(1.12±0.10)×10 -12,ķ 4(Ť)=(1.80±0.26)×10 -11 EXP( - (816±43)/ Ť)。发现环状分子比具有相同数量的-CH 3基团的类似线性分子的反应性低,而线性和环状全甲基硅氧烷的反应性都随CH 3-基团数量的增加而增加。将当前结果与以前的速率系数确定(如果有)进行比较。本研究中包括的全甲基硅氧烷是大气中短寿命的化合物,估计的大气寿命分别为11天,8天,17天和13天。
更新日期:2018-04-06
中文翻译:

OH自由基与线性(L 2,L 3)和环状(D 3,D 4)全甲基硅氧烷的气相反应的温度相关速率系数
在生产过程中,全甲基硅氧烷会排放到大气中,并用作个人护理产品,润滑剂和清洁剂。预期全甲基硅氧烷的主要大气损失过程是通过与OH自由基的气相反应。在这项研究中,使用脉冲激光光解-激光诱导荧光技术在240-370 K和温度范围内,用两种最简单的线性和环状全甲基硅氧烷对OH自由基气相反应的速率系数k(T)进行了测量。在294K下的相对速率法:六甲基二硅氧烷((CH 3)3 SiOSi(CH 3)3,L 2),k 1;八甲基三硅氧烷([(CH 3)3 SiO] 2 Si(CH 3)2,L 3),k 2;六甲基环三硅氧烷([-Si(CH 3)2 O-] 3,D 3),k 3;和八甲基环四硅氧烷([-Si(CH 3)2 O-] 4,D 4),k 4。对于240–370 K的温度范围,获得的k(294 K)值和温度依赖性表达式为(cm 3分子–1 s –1,2σ绝对不确定性):k 1(294 K)=(1.28±0.08)×10 –12,k 1(T)=(1.87±0.18)×10 –11 exp(-(791±27)/ T); k 2(294 K)=(1.72±0.10)×10 –12,k 2(T)= 1.96×10 –13(T / 298)4.34 exp(657 / T); k 3(294 K)=(0.82±0.05)×10 –12,k 3(T)=(1.29±0.19)×10 –11 exp(-(805±43)/ T); 和ķ 4(294 K)=(1.12±0.10)×10 -12,ķ 4(Ť)=(1.80±0.26)×10 -11 EXP( - (816±43)/ Ť)。发现环状分子比具有相同数量的-CH 3基团的类似线性分子的反应性低,而线性和环状全甲基硅氧烷的反应性都随CH 3-基团数量的增加而增加。将当前结果与以前的速率系数确定(如果有)进行比较。本研究中包括的全甲基硅氧烷是大气中短寿命的化合物,估计的大气寿命分别为11天,8天,17天和13天。











































 京公网安备 11010802027423号
京公网安备 11010802027423号