当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An unprecedented tandem synthesis of fluorescent coumarin-fused pyrimidines via copper-catalyzed cross-dehydrogenative C(sp3)–N bond coupling†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8ob00586a Santosh Kumari 1, 2, 3, 4 , S. M. Abdul Shakoor 1, 2, 3, 4 , Sadhika Khullar 1, 4, 5, 6, 7 , Sanjay K. Mandal 4, 8, 9 , Rajeev Sakhuja 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8ob00586a Santosh Kumari 1, 2, 3, 4 , S. M. Abdul Shakoor 1, 2, 3, 4 , Sadhika Khullar 1, 4, 5, 6, 7 , Sanjay K. Mandal 4, 8, 9 , Rajeev Sakhuja 1, 2, 3, 4
Affiliation
|
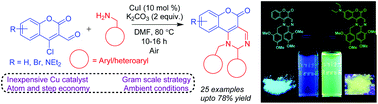
An efficient, one-pot Cu-catalyzed tandem synthesis of fluorescent 1-benzyl-2-phenyl-1,2-dihydro-5H-chromeno[4,3-d]pyrimidin-5-ones from 4-chloro-3-formylcoumarin and benzylamines was developed by in situ intramolecular cross-dehydrogenative C(sp3)–N bond formation in moderate-to-good yields under ligand-free ambient conditions. This synthesis was easily scalable, and the generality of the substrates was established. These coumarin-fused pyrimidines exhibited interesting photo-physical properties and high quantum yields, and would be potential candidates for facilitating suitable studies in medicinal chemistry and materials science.
中文翻译:

通过铜催化的交叉脱氢C(sp 3)–N键偶联, 空前串联合成荧光香豆素融合的嘧啶†
有效的一锅铜催化串联合成从4-氯-3-荧光的1-苄基-2-苯基-1,2-二氢-5 H-苯并[4,3 - d ]嘧啶-5-酮甲酰基香豆素和苄胺是通过在无配体的环境条件下以中等至良好的产率原位形成分子内交叉脱氢的C(sp 3)–N键形成的。该合成易于扩展,并建立了底物的通用性。这些香豆素融合的嘧啶显示出令人感兴趣的光物理性质和高量子产率,并且可能是促进药物化学和材料科学中的适当研究的潜在候选者。
更新日期:2018-04-05
中文翻译:

通过铜催化的交叉脱氢C(sp 3)–N键偶联, 空前串联合成荧光香豆素融合的嘧啶†
有效的一锅铜催化串联合成从4-氯-3-荧光的1-苄基-2-苯基-1,2-二氢-5 H-苯并[4,3 - d ]嘧啶-5-酮甲酰基香豆素和苄胺是通过在无配体的环境条件下以中等至良好的产率原位形成分子内交叉脱氢的C(sp 3)–N键形成的。该合成易于扩展,并建立了底物的通用性。这些香豆素融合的嘧啶显示出令人感兴趣的光物理性质和高量子产率,并且可能是促进药物化学和材料科学中的适当研究的潜在候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号