当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent advances in radical-based C–N bond formation via photo-/electrochemistry
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c7cs00572e Yating Zhao 1, 2, 3, 4, 5 , Wujiong Xia 1, 2, 3, 4
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c7cs00572e Yating Zhao 1, 2, 3, 4, 5 , Wujiong Xia 1, 2, 3, 4
Affiliation
|
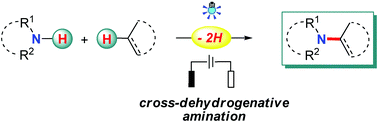
The employment of nitrogen sources with free N–H bonds for amination is considered to be most straightforward and desirable, especially when the C–N bonds are prepared from N–H bonds and non-functionalized carbon sources, such as C–H bonds and C–C double/triple bonds, since this obviates the needs for the pre-installation of reactive groups in the starting materials and leads to a high atom and step economy. Recently, radical chemistry has been resuscitated owing to its great value in organic synthesis, and notable advances have been made in the direct use of N–H bonds for radical-based C–N bond formation with photo-/electrotechniques. Apart from the well-studied N-radical species addition pathway, radical-mediated aminations also proceed through N-atom nucleophilic addition, C-/N-radical cross-coupling, and a hydrogen-atom transfer (HAT) process. This review highlights the recent advances in this area with emphasis on the related reaction mechanisms.
中文翻译:

通过光/电化学 形成基于自由基的C–N键的最新进展
认为使用带有自由N–H键的氮源进行胺化是最直接和最理想的方法,尤其是当C–N键是由N–H键和非功能化碳源(例如C–H键和C–C双键/三键,因为这消除了在原料中预先安装反应基的需要,并带来了高原子和阶梯经济性。近年来,自由基化学因其在有机合成中的巨大价值而得到了复兴,在将N–H键直接用于光/电子技术形成基于自由基的C–N键方面,已经取得了显着进展。除了经过充分研究的N自由基物种加成途径外,自由基介导的胺化反应还通过N原子亲核加成,C- / N自由基交叉偶联和氢原子转移(HAT)过程进行。
更新日期:2018-04-05
中文翻译:

通过光/电化学 形成基于自由基的C–N键的最新进展
认为使用带有自由N–H键的氮源进行胺化是最直接和最理想的方法,尤其是当C–N键是由N–H键和非功能化碳源(例如C–H键和C–C双键/三键,因为这消除了在原料中预先安装反应基的需要,并带来了高原子和阶梯经济性。近年来,自由基化学因其在有机合成中的巨大价值而得到了复兴,在将N–H键直接用于光/电子技术形成基于自由基的C–N键方面,已经取得了显着进展。除了经过充分研究的N自由基物种加成途径外,自由基介导的胺化反应还通过N原子亲核加成,C- / N自由基交叉偶联和氢原子转移(HAT)过程进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号