当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereoisomers of l-proline-linked trityl-nitroxide biradicals: synthesis and effect of chiral configurations on exchange interactions†
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8sc00969d Weixiang Zhai 1, 2, 3, 4, 5 , Yalan Feng 1, 2, 3, 4, 5 , Huiqiang Liu 1, 2, 3, 4, 5 , Antal Rockenbauer 6, 7, 8, 9, 10 , Deni Mance 11, 12, 13, 14, 15 , Shaoyong Li 1, 2, 3, 4, 5 , Yuguang Song 1, 2, 3, 4, 5 , Marc Baldus 11, 12, 13, 14, 15 , Yangping Liu 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1039/c8sc00969d Weixiang Zhai 1, 2, 3, 4, 5 , Yalan Feng 1, 2, 3, 4, 5 , Huiqiang Liu 1, 2, 3, 4, 5 , Antal Rockenbauer 6, 7, 8, 9, 10 , Deni Mance 11, 12, 13, 14, 15 , Shaoyong Li 1, 2, 3, 4, 5 , Yuguang Song 1, 2, 3, 4, 5 , Marc Baldus 11, 12, 13, 14, 15 , Yangping Liu 1, 2, 3, 4, 5
Affiliation
|
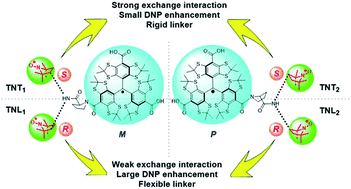
The exchange (J) interaction of organic biradicals is a crucial factor controlling their physiochemical properties and potential applications and can be modulated by changing the nature of the linker. In the present work, we for the first time demonstrate the effect of chiral configurations of radical parts on the J values of trityl-nitroxide (TN) biradicals. Four diastereoisomers (TNT1, TNT2, TNL1 and TNL2) of TN biradicals were synthesized and purified by the conjugation of a racemic (R/S) nitroxide with the racemic (M/P) trityl radical viaL-proline. The absolute configurations of these diastereoisomers were assigned by comparing experimental and calculated electronic circular dichroism (ECD) spectra as (M, S, S) for TNT1, (P, S, S) for TNT2, (M, S, R) for TNL1 and (P, S, R) for TNL2. Electron paramagnetic resonance (EPR) results showed that the configuration of the nitroxide part instead of the trityl part is dominant in controlling the exchange interactions and the order of the J values at room temperature is TNT1 (252 G) > TNT2 (127 G) ≫ TNL2 (33 G) > TNL1 (14 G). Moreover, the J values of TNL1/TNL2 with the S configuration in the nitroxide part vary with temperature and the polarity of solvents due to their flexible linker, whereas the J values of TNT1/TNT2 are almost insensitive to these two factors due to the rigidity of their linkers. The distinct exchange interactions between TNT1,2 and TNL1,2 in the frozen state led to strongly different high-field dynamic nuclear polarization (DNP) enhancements with ε = 7 for TNT1,2 and 40 for TNL1,2 under 800 MHz DNP conditions.
中文翻译:

l-脯氨酸连接的三苯甲基-氮氧杂双自由基的 非对映异构体:手性构型的合成及其对交换相互作用的影响†
有机双自由基的交换(J)相互作用是控制其理化性质和潜在应用的关键因素,可以通过改变接头的性质进行调节。在目前的工作中,我们第一次证明自由基部分的手性构型对三苯甲基-硝基氧(TN)双自由基的J值的影响。合成和纯化了TN双自由基的四种非对映异构体(TNT 1,TNT 2,TNL 1和TNL 2),通过外消旋(R / S)一氧化氮与外消旋(M / P)三苯甲基自由基通过L的共轭纯化。-脯氨酸。这些非对映体的绝对构型通过比较实验和计算电子圆二色性(ECD)光谱为(分配中号,小号,小号用于TNT)1,(P,小号,小号),用于TNT 2,(中号,小号,- [R )对于TNL 1和(P,S,R)对于TNL 2。电子顺磁共振(EPR)结果表明,氮氧自由基部分而非三苯甲基部分的构型在控制交换相互作用中占主导地位,室温下J值的顺序为TNT 1(252 G)> TNT 2(127 G) )≫ TNL 2(33 G)> TNL 1(14 G)。此外,由于其柔性连接基,氮氧化物部分中具有S构型的TNL 1 / TNL 2的J值随温度和溶剂极性而变化,而TNT 1 / TNT 2的J值则随它们的变化而变化。由于连接子的刚性,它们对这两个因素几乎不敏感。冷冻状态下TNT 1,2和TNL 1,2之间明显的交换相互作用导致了高场动态核极化(DNP)的增强,在800以下时,TNT 1,2的ε = 7 ,TNL 1,2的ε = 40 MHz DNP条件。
更新日期:2018-04-05
中文翻译:

l-脯氨酸连接的三苯甲基-氮氧杂双自由基的 非对映异构体:手性构型的合成及其对交换相互作用的影响†
有机双自由基的交换(J)相互作用是控制其理化性质和潜在应用的关键因素,可以通过改变接头的性质进行调节。在目前的工作中,我们第一次证明自由基部分的手性构型对三苯甲基-硝基氧(TN)双自由基的J值的影响。合成和纯化了TN双自由基的四种非对映异构体(TNT 1,TNT 2,TNL 1和TNL 2),通过外消旋(R / S)一氧化氮与外消旋(M / P)三苯甲基自由基通过L的共轭纯化。-脯氨酸。这些非对映体的绝对构型通过比较实验和计算电子圆二色性(ECD)光谱为(分配中号,小号,小号用于TNT)1,(P,小号,小号),用于TNT 2,(中号,小号,- [R )对于TNL 1和(P,S,R)对于TNL 2。电子顺磁共振(EPR)结果表明,氮氧自由基部分而非三苯甲基部分的构型在控制交换相互作用中占主导地位,室温下J值的顺序为TNT 1(252 G)> TNT 2(127 G) )≫ TNL 2(33 G)> TNL 1(14 G)。此外,由于其柔性连接基,氮氧化物部分中具有S构型的TNL 1 / TNL 2的J值随温度和溶剂极性而变化,而TNT 1 / TNT 2的J值则随它们的变化而变化。由于连接子的刚性,它们对这两个因素几乎不敏感。冷冻状态下TNT 1,2和TNL 1,2之间明显的交换相互作用导致了高场动态核极化(DNP)的增强,在800以下时,TNT 1,2的ε = 7 ,TNL 1,2的ε = 40 MHz DNP条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号