当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anomalous H2 Desorption Rate of NaAlH4 Confined in Nitrogen-Doped Nanoporous Carbon Frameworks
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1021/acs.chemmater.8b00305 Christopher L. Carr , Waruni Jayawardana , Hongyang Zou 1 , James L. White 2 , Farid El Gabaly 2 , Mark S. Conradi 1, 3 , Vitalie Stavila 2 , Mark D. Allendorf 2 , Eric H. Majzoub
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1021/acs.chemmater.8b00305 Christopher L. Carr , Waruni Jayawardana , Hongyang Zou 1 , James L. White 2 , Farid El Gabaly 2 , Mark S. Conradi 1, 3 , Vitalie Stavila 2 , Mark D. Allendorf 2 , Eric H. Majzoub
Affiliation
|
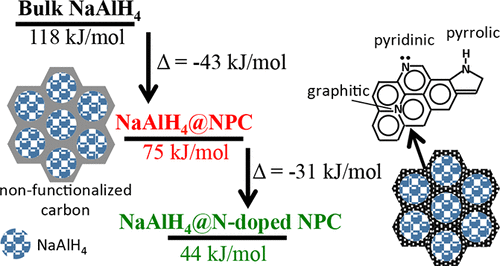
Confining NaAlH4 in nanoporous carbon scaffolds is known to alter the sorption kinetics and/or pathways of the characteristic bulk hydride reactions through interaction with the framework at the interface, increased specific surface area of the resulting nanoparticles, decreased hydrogen diffusion distances, and prevention of phase segregation. Although the nanosize effects have been well studied, the influence of the carbon scaffold surface chemistry remains unclear. Here we compare the hydrogen sorption characteristics of NaAlH4 confined by melt infiltration in nitrogen-doped/undoped ordered nanoporous carbon of two different geometries. 23Na and 27Al MAS NMR, N2 sorption, and PXRD verify NaAlH4 was successfully confined and remains intact in the carbon nanopores after infiltration. Both the N-doped/undoped nanoconfined systems demonstrate improved reversibility in relation to the bulk hydride during hydrogen desorption/absorption cycling. Isothermal kinetic measurements indicate a lowering of the activation energy for H2 desorption by as much as 70 kJ/mol in N-doped frameworks, far larger than the reduction in carbon-only frameworks. Most interestingly, this dramatic lowering of the activation energy is accompanied by an unexpected and anomalously low NaAlH4 desorption rate in the N-doped frameworks. This suggests that the framework surface chemistry plays an important role in the desorption process and that the rate limiting step for desorption may be associated with interactions of the hydride and host surface. Our results indicate that functionalization of carbon scaffold surface chemistry with heteroatoms provides a powerful method of altering the characteristic hydrogen sorption properties of confined metal hydride systems. This technique may prove beneficial in the path to a viable metal hydride-based hydrogen storage system.
中文翻译:

氮掺杂纳米多孔碳骨架中NaAlH 4的异常H 2解吸速率
已知将NaAlH 4限制在纳米多孔碳支架中可通过与界面处的骨架相互作用,增加所得纳米颗粒的比表面积,减少氢扩散距离并防止氢原子转移来改变特征性本体氢化物反应的吸附动力学和/或途径。相分离。尽管已经很好地研究了纳米尺寸的影响,但是碳支架表面化学的影响仍然不清楚。在这里,我们比较了两种不同几何形状的氮掺杂/非掺杂有序纳米多孔碳中熔体渗透限制的NaAlH 4的氢吸附特性。23 Na和27 Al MAS NMR,N 2吸附和PXRD验证NaAlH4被成功地限制,并在渗透后在碳纳米孔中保持完好无损。相对于本体氢化物,在氢解吸/吸收循环中,N掺杂/未掺杂的纳米限制体系都表现出改善的可逆性。等温动力学测量表明,在氮掺杂骨架中,H 2解吸的活化能降低了多达70 kJ / mol,远大于仅碳骨架的降低。最有趣的是,活化能的急剧降低伴随着NaAlH 4的异常降低和异常降低氮掺杂骨架中的解吸速率。这表明骨架表面化学在解吸过程中起着重要作用,并且解吸的限速步骤可能与氢化物和主体表面的相互作用有关。我们的结果表明,用杂原子对碳支架表面化学进行功能化提供了一种强大的方法,可改变受限金属氢化物系统的特征氢吸附特性。在通往可行的基于金属氢化物的储氢系统的过程中,该技术可能被证明是有益的。
更新日期:2018-04-04
中文翻译:

氮掺杂纳米多孔碳骨架中NaAlH 4的异常H 2解吸速率
已知将NaAlH 4限制在纳米多孔碳支架中可通过与界面处的骨架相互作用,增加所得纳米颗粒的比表面积,减少氢扩散距离并防止氢原子转移来改变特征性本体氢化物反应的吸附动力学和/或途径。相分离。尽管已经很好地研究了纳米尺寸的影响,但是碳支架表面化学的影响仍然不清楚。在这里,我们比较了两种不同几何形状的氮掺杂/非掺杂有序纳米多孔碳中熔体渗透限制的NaAlH 4的氢吸附特性。23 Na和27 Al MAS NMR,N 2吸附和PXRD验证NaAlH4被成功地限制,并在渗透后在碳纳米孔中保持完好无损。相对于本体氢化物,在氢解吸/吸收循环中,N掺杂/未掺杂的纳米限制体系都表现出改善的可逆性。等温动力学测量表明,在氮掺杂骨架中,H 2解吸的活化能降低了多达70 kJ / mol,远大于仅碳骨架的降低。最有趣的是,活化能的急剧降低伴随着NaAlH 4的异常降低和异常降低氮掺杂骨架中的解吸速率。这表明骨架表面化学在解吸过程中起着重要作用,并且解吸的限速步骤可能与氢化物和主体表面的相互作用有关。我们的结果表明,用杂原子对碳支架表面化学进行功能化提供了一种强大的方法,可改变受限金属氢化物系统的特征氢吸附特性。在通往可行的基于金属氢化物的储氢系统的过程中,该技术可能被证明是有益的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号