Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structures of the gastric proton pump
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0003-8 Kazuhiro Abe , Katsumasa Irie , Hanayo Nakanishi , Hiroshi Suzuki , Yoshinori Fujiyoshi
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0003-8 Kazuhiro Abe , Katsumasa Irie , Hanayo Nakanishi , Hiroshi Suzuki , Yoshinori Fujiyoshi
|
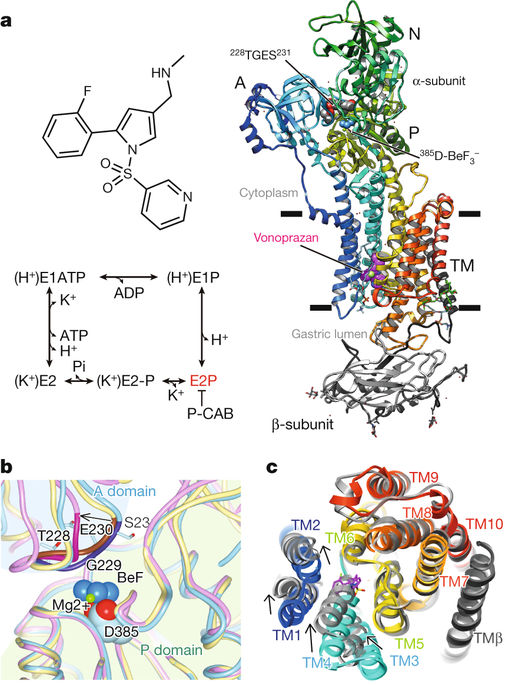
The gastric proton pump—the H+, K+-ATPase—is a P-type ATPase responsible for acidifying the gastric juice down to pH 1. This corresponds to a million-fold proton gradient across the membrane of the parietal cell, the steepest known cation gradient of any mammalian tissue. The H+, K+-ATPase is an important target for drugs that treat gastric acid-related diseases. Here we present crystal structures of the H+, K+-ATPase in complex with two blockers, vonoprazan and SCH28080, in the luminal-open state, at 2.8 Å resolution. The drugs have partially overlapping but clearly distinct binding modes in the middle of a conduit running from the gastric lumen to the cation-binding site. The crystal structures suggest that the tight configuration at the cation-binding site lowers the pKa value of Glu820 sufficiently to enable the release of a proton even into the pH 1 environment of the stomach.Crystal structures of the gastric proton pump in complex with two inhibitory drugs reveal the mechanism that generates the steep acidic gradient across the membranes of parietal cells.
中文翻译:

胃质子泵的晶体结构
胃质子泵——H+、K+-ATP 酶——是一种 P 型 ATP 酶,负责将胃液酸化至 pH 1。这对应于壁细胞膜上的百万倍质子梯度,这是已知的最陡峭的阳离子任何哺乳动物组织的梯度。H+、K+-ATPase 是治疗胃酸相关疾病药物的重要靶点。在这里,我们展示了 H+、K+-ATPase 与两种阻滞剂 vonoprazan 和 SCH28080 复合物的晶体结构,处于管腔开放状态,分辨率为 2.8 Å。这些药物在从胃腔到阳离子结合位点的管道中间具有部分重叠但明显不同的结合模式。
更新日期:2018-04-01
中文翻译:

胃质子泵的晶体结构
胃质子泵——H+、K+-ATP 酶——是一种 P 型 ATP 酶,负责将胃液酸化至 pH 1。这对应于壁细胞膜上的百万倍质子梯度,这是已知的最陡峭的阳离子任何哺乳动物组织的梯度。H+、K+-ATPase 是治疗胃酸相关疾病药物的重要靶点。在这里,我们展示了 H+、K+-ATPase 与两种阻滞剂 vonoprazan 和 SCH28080 复合物的晶体结构,处于管腔开放状态,分辨率为 2.8 Å。这些药物在从胃腔到阳离子结合位点的管道中间具有部分重叠但明显不同的结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号