Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular nucleation mechanisms and control strategies for crystal polymorph selection
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/nature25971 Alexander E. S. Van Driessche , Nani Van Gerven , Paul H. H. Bomans , Rick R. M. Joosten , Heiner Friedrich , David Gil-Carton , Nico A. J. M. Sommerdijk , Mike Sleutel
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/nature25971 Alexander E. S. Van Driessche , Nani Van Gerven , Paul H. H. Bomans , Rick R. M. Joosten , Heiner Friedrich , David Gil-Carton , Nico A. J. M. Sommerdijk , Mike Sleutel
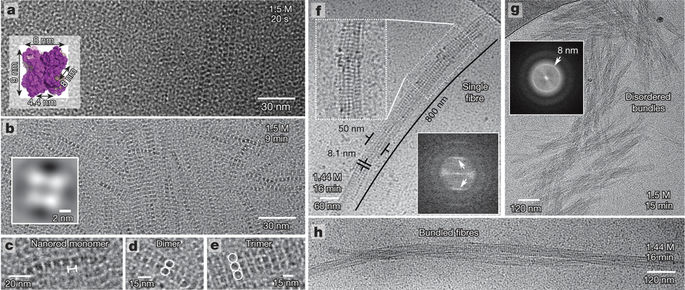
|
The formation of condensed (compacted) protein phases is associated with a wide range of human disorders, such as eye cataracts, amyotrophic lateral sclerosis, sickle cell anaemia and Alzheimer’s disease. However, condensed protein phases have their uses: as crystals, they are harnessed by structural biologists to elucidate protein structures, or are used as delivery vehicles for pharmaceutical applications. The physiochemical properties of crystals can vary substantially between different forms or structures (‘polymorphs’) of the same macromolecule, and dictate their usability in a scientific or industrial context. To gain control over an emerging polymorph, one needs a molecular-level understanding of the pathways that lead to the various macroscopic states and of the mechanisms that govern pathway selection. However, it is still not clear how the embryonic seeds of a macromolecular phase are formed, or how these nuclei affect polymorph selection. Here we use time-resolved cryo-transmission electron microscopy to image the nucleation of crystals of the protein glucose isomerase, and to uncover at molecular resolution the nucleation pathways that lead to two crystalline states and one gelled state. We show that polymorph selection takes place at the earliest stages of structure formation and is based on specific building blocks for each space group. Moreover, we demonstrate control over the system by selectively forming desired polymorphs through site-directed mutagenesis, specifically tuning intermolecular bonding or gel seeding. Our results differ from the present picture of protein nucleation, in that we do not identify a metastable dense liquid as the precursor to the crystalline state. Rather, we observe nucleation events that are driven by oriented attachments between subcritical clusters that already exhibit a degree of crystallinity. These insights suggest ways of controlling macromolecular phase transitions, aiding the development of protein-based drug-delivery systems and macromolecular crystallography.
中文翻译:

晶体多晶型选择的分子成核机制和控制策略
凝聚(压缩)蛋白相的形成与多种人类疾病有关,例如眼白内障、肌萎缩侧索硬化、镰状细胞性贫血和阿尔茨海默病。然而,凝聚的蛋白质相有其用途:作为晶体,它们被结构生物学家用来阐明蛋白质结构,或用作药物应用的传递载体。晶体的理化性质在同一大分子的不同形式或结构(“多晶型”)之间可能会有很大差异,并决定了它们在科学或工业环境中的可用性。为了控制新出现的多晶型物,人们需要对导致各种宏观状态的途径和控制途径选择的机制有分子水平的理解。然而,目前尚不清楚大分子相的胚胎种子是如何形成的,或者这些细胞核如何影响多晶型选择。在这里,我们使用时间分辨低温透射电子显微镜对蛋白质葡萄糖异构酶的晶体成核进行成像,并以分子分辨率揭示导致两种结晶状态和一种凝胶状态的成核途径。我们表明多晶型选择发生在结构形成的最早阶段,并且基于每个空间群的特定构建块。此外,我们通过定点诱变选择性地形成所需的多晶型物来证明对系统的控制,特别是调整分子间键合或凝胶接种。我们的结果不同于目前蛋白质成核的图片,因为我们没有将亚稳态致密液体识别为结晶状态的前体。相反,我们观察到成核事件是由已经表现出一定程度结晶度的亚临界簇之间的定向附着驱动的。这些见解提出了控制大分子相变的方法,有助于开发基于蛋白质的药物输送系统和大分子晶体学。
更新日期:2018-04-01
中文翻译:

晶体多晶型选择的分子成核机制和控制策略
凝聚(压缩)蛋白相的形成与多种人类疾病有关,例如眼白内障、肌萎缩侧索硬化、镰状细胞性贫血和阿尔茨海默病。然而,凝聚的蛋白质相有其用途:作为晶体,它们被结构生物学家用来阐明蛋白质结构,或用作药物应用的传递载体。晶体的理化性质在同一大分子的不同形式或结构(“多晶型”)之间可能会有很大差异,并决定了它们在科学或工业环境中的可用性。为了控制新出现的多晶型物,人们需要对导致各种宏观状态的途径和控制途径选择的机制有分子水平的理解。然而,目前尚不清楚大分子相的胚胎种子是如何形成的,或者这些细胞核如何影响多晶型选择。在这里,我们使用时间分辨低温透射电子显微镜对蛋白质葡萄糖异构酶的晶体成核进行成像,并以分子分辨率揭示导致两种结晶状态和一种凝胶状态的成核途径。我们表明多晶型选择发生在结构形成的最早阶段,并且基于每个空间群的特定构建块。此外,我们通过定点诱变选择性地形成所需的多晶型物来证明对系统的控制,特别是调整分子间键合或凝胶接种。我们的结果不同于目前蛋白质成核的图片,因为我们没有将亚稳态致密液体识别为结晶状态的前体。相反,我们观察到成核事件是由已经表现出一定程度结晶度的亚临界簇之间的定向附着驱动的。这些见解提出了控制大分子相变的方法,有助于开发基于蛋白质的药物输送系统和大分子晶体学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号