当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Surface Structural Recognition in Inactive Areas: A New Immobilization Strategy for Acetylcholinesterase
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00160 Jianxiong Diao 1 , Xiaolu Yu 1 , Lin Ma 2 , Yuanqing Li 1 , Ying Sun 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00160 Jianxiong Diao 1 , Xiaolu Yu 1 , Lin Ma 2 , Yuanqing Li 1 , Ying Sun 1
Affiliation
|
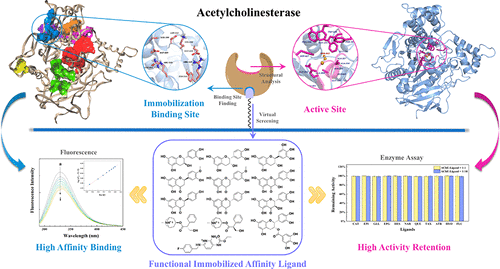
This work reported a new method of design for the immobilization of acetylcholinesterase (AChE) based on its molecular structure to improve its sensitivity and stability. The immobilization binding site on the surface of AChE was determined using MOLCAD’s multi-channel functionality. Then, 11 molecules ((+)-catechin, (−)-epicatechin, (−)-gallocatechin, hesperetin, naringenin, quercetin, taxifolin, (−)-epicatechin gallate, flupirtine, atropine, and hyoscyamine) were selected from the ZINC database (about 50 000 molecules) as candidate affinity ligands for AChE. The fluorescence results showed that the binding constant Kb between AChE and the ligands ranged from 0.01344 × 104 to 4.689 × 104 M–1 and there was one independent class of binding site for the ligands on AChE. The AChE-ligand binding free energy ranged from −12.14 to −26.65 kJ mol–1. Naringenin, hesperetin, and quercetin were the three most potent immobilized affinity ligands. In addition, it was confirmed that the binding between the immobilized ligands only occurred at a single site, located in an inactive area on the surface of AChE, and did not affect the enzymatic activity as shown through a competition experiment and enzyme assay. This method based on protein surface structural recognition with high sensitivity and stability can be used as a generic approach for design of the enzyme immobilization and biosensor development.
中文翻译:

蛋白质在非活性区域的表面结构识别:乙酰胆碱酯酶的一种新的固定化策略
这项工作报告了一种新的固定化乙酰胆碱酯酶(AChE)分子结构的设计方法,以提高其敏感性和稳定性。使用MOLCAD的多通道功能确定了AChE表面的固定结合位点。然后,从ZINC中选择了11种分子((+)-儿茶素,(-)-表儿茶素,(-)-加洛茶素,橙皮素,柚皮素,槲皮素,滑石粉,(-)-表儿茶素没食子酸酯,氟吡汀,阿托品和hyoscyamine)数据库(约5万个分子)作为AChE的候选亲和配体。荧光结果表明,AChE与配体之间的结合常数K b为0.01344×10 4至4.689×10 4 M –1AChE上的配体有一类独立的结合位点。AChE-配体结合自由能的范围为-12.14至-26.65 kJ mol –1。柚皮素,橙皮素和槲皮素是三种最有效的固定化亲和配体。另外,证实了固定的配体之间的结合仅发生在位于AChE表面上的非活性区域中的单个位点,并且不影响通过竞争实验和酶测定法显示的酶活性。这种基于蛋白质表面结构识别的方法具有很高的灵敏度和稳定性,可以用作酶固定化设计和生物传感器开发的通用方法。
更新日期:2018-04-04
中文翻译:

蛋白质在非活性区域的表面结构识别:乙酰胆碱酯酶的一种新的固定化策略
这项工作报告了一种新的固定化乙酰胆碱酯酶(AChE)分子结构的设计方法,以提高其敏感性和稳定性。使用MOLCAD的多通道功能确定了AChE表面的固定结合位点。然后,从ZINC中选择了11种分子((+)-儿茶素,(-)-表儿茶素,(-)-加洛茶素,橙皮素,柚皮素,槲皮素,滑石粉,(-)-表儿茶素没食子酸酯,氟吡汀,阿托品和hyoscyamine)数据库(约5万个分子)作为AChE的候选亲和配体。荧光结果表明,AChE与配体之间的结合常数K b为0.01344×10 4至4.689×10 4 M –1AChE上的配体有一类独立的结合位点。AChE-配体结合自由能的范围为-12.14至-26.65 kJ mol –1。柚皮素,橙皮素和槲皮素是三种最有效的固定化亲和配体。另外,证实了固定的配体之间的结合仅发生在位于AChE表面上的非活性区域中的单个位点,并且不影响通过竞争实验和酶测定法显示的酶活性。这种基于蛋白质表面结构识别的方法具有很高的灵敏度和稳定性,可以用作酶固定化设计和生物传感器开发的通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号