当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of new 3-amino-2-azetidinone derivatives as anti-colorectal cancer agents†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8md00147b Farida Tripodi 1, 2, 3, 4 , Federico Dapiaggi 4, 5, 6, 7 , Fulvia Orsini 4, 5, 6, 7 , Roberto Pagliarin 4, 5, 6, 7 , Guido Sello 4, 5, 6, 7 , Paola Coccetti 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8md00147b Farida Tripodi 1, 2, 3, 4 , Federico Dapiaggi 4, 5, 6, 7 , Fulvia Orsini 4, 5, 6, 7 , Roberto Pagliarin 4, 5, 6, 7 , Guido Sello 4, 5, 6, 7 , Paola Coccetti 1, 2, 3, 4
Affiliation
|
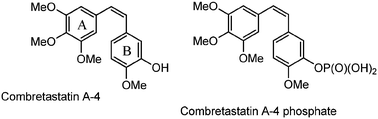
Several synthetic combretastatin A4 (CA-4) derivatives were recently prepared to increase the drug efficacy and stability of the natural product isolated from the South African tree Combretum caffrum. A group of ten 3-amino-2-azetidinone derivatives, as combretastatin A4 analogues, was selected through docking experiments, synthesized and tested for their anti-proliferative activity against the colon cancer SW48 cell line. These molecules, through the formation of amide bonds in position 3, allow the synthesis of various derivatives that can modulate the activity with great resistance to hydrolytic conditions. The cyclization to obtain the 3-aminoazetidinone ring is highly diastereoselective and provides a trans biologically active isomer under mild reaction conditions with better yields than the 3-hydroxy-2-azetidinone synthesis. All compounds showed IC50 values ranging between 14.0 and 564.2 nM, and the most active compound showed inhibitory activity against tubulin polymerization in vitro, being a potential therapeutic agent against colon cancer.
中文翻译:

新型3-氨基-2-氮杂环丁酮衍生物作为抗结直肠癌药物的合成和生物学评估†
最近制备了几种合成的康伐他汀A4(CA-4)衍生物,以提高从南非树Com bre中分离的天然产物的药效和稳定性。通过对接实验选择了一组十种3-氨基-2-氮杂环丁酮衍生物,作为康普他汀A4类似物,进行了合成并测试了它们对结肠癌SW48细胞系的抗增殖活性。这些分子通过在位置3上形成酰胺键,可以合成各种衍生物,这些衍生物可以调节活性,并且对水解条件的抵抗力强。获得3-氨基氮杂环丁酮环的环化是非对映选择性高的,并提供反式生物活性异构体在温和的反应条件下具有比3-羟基-2-氮杂环丁酮合成更高的收率。所有化合物的IC 50值均在14.0至564.2 nM之间,活性最高的化合物在体外显示出对微管蛋白聚合的抑制活性,是抗结肠癌的潜在治疗剂。
更新日期:2018-04-04
中文翻译:

新型3-氨基-2-氮杂环丁酮衍生物作为抗结直肠癌药物的合成和生物学评估†
最近制备了几种合成的康伐他汀A4(CA-4)衍生物,以提高从南非树Com bre中分离的天然产物的药效和稳定性。通过对接实验选择了一组十种3-氨基-2-氮杂环丁酮衍生物,作为康普他汀A4类似物,进行了合成并测试了它们对结肠癌SW48细胞系的抗增殖活性。这些分子通过在位置3上形成酰胺键,可以合成各种衍生物,这些衍生物可以调节活性,并且对水解条件的抵抗力强。获得3-氨基氮杂环丁酮环的环化是非对映选择性高的,并提供反式生物活性异构体在温和的反应条件下具有比3-羟基-2-氮杂环丁酮合成更高的收率。所有化合物的IC 50值均在14.0至564.2 nM之间,活性最高的化合物在体外显示出对微管蛋白聚合的抑制活性,是抗结肠癌的潜在治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号