当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of far-infrared spectroscopy to the structural identification of protein materials
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8cp00802g Yanchen Han 1, 2, 3 , Shengjie Ling 2, 3, 4 , Zeming Qi 3, 5, 6 , Zhengzhong Shao 1, 2, 3 , Xin Chen 1, 2, 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8cp00802g Yanchen Han 1, 2, 3 , Shengjie Ling 2, 3, 4 , Zeming Qi 3, 5, 6 , Zhengzhong Shao 1, 2, 3 , Xin Chen 1, 2, 3
Affiliation
|
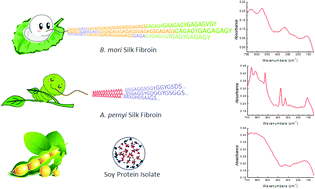
Although far-infrared (IR) spectroscopy has been shown to be a powerful tool to determine peptide structure and to detect structural transitions in peptides, it has been overlooked in the characterization of proteins. Herein, we used far-IR spectroscopy to monitor the structure of four abundant non-bioactive proteins, namely, soybean protein isolate (SPI), pea protein isolate (PPI) and two types of silk fibroins (SFs), domestic Bombyx mori and wild Antheraea pernyi. The two globular proteins SPI and PPI result in broad and weak far-IR bands (between 50 and 700 cm−1), in agreement with those of some other bioactive globular proteins previously studied (lysozyme, myoglobin, hemoglobin, etc.) that generally only have random amino acid sequences. Interestingly, the two SFs, which are characterized by a structure composed of highly repetitive motifs, show several sharp far-IR characteristic absorption peaks. Moreover, some of these characteristic peaks (such as the peaks at 260 and 428 cm−1 in B. mori, and the peaks at 245 and 448 cm−1 in A. pernyi) are sensitive to conformational changes; hence, they can be directly used to monitor conformational transitions in SFs. Furthermore, since SF absorption bands clearly differ from those of globular proteins and different SFs even show distinct adsorption bands, far-IR spectroscopy can be applied to distinguish and determine the specific SF component within protein blends.
中文翻译:

远红外光谱技术在蛋白质材料结构鉴定中的应用
尽管已证明远红外(IR)光谱是确定肽结构和检测肽中结构转变的强大工具,但在蛋白质表征中却被忽略了。在这里,我们使用远红外光谱法来监测四种丰富的非生物活性蛋白的结构,分别是大豆蛋白分离物(SPI),豌豆蛋白分离物(PPI)和两种类型的丝素蛋白(SF),即家蚕和野生蚕丝蛋白。ther药。与先前研究的其他一些生物活性球蛋白(溶菌酶,肌红蛋白,血红蛋白等)一致,两种球形蛋白SPI和PPI导致宽和弱的远红外波段(介于50和700 cm -1之间)。),通常只有随机的氨基酸序列。有趣的是,这两个SF的特征是由高度重复的基序组成的结构显示出几个尖锐的远红外特征吸收峰。而且,这些特征峰中的一些(例如,在桑蚕中的260和428cm -1处的峰,以及在百日草中的245和448cm -1处的峰)对构象变化敏感。因此,它们可以直接用于监测SF中的构象转变。此外,由于SF吸收带明显不同于球形蛋白,并且不同的SF甚至显示出不同的吸收带,因此可以使用远红外光谱法来区分和确定蛋白质混合物中的特定SF成分。
更新日期:2018-04-04
中文翻译:

远红外光谱技术在蛋白质材料结构鉴定中的应用
尽管已证明远红外(IR)光谱是确定肽结构和检测肽中结构转变的强大工具,但在蛋白质表征中却被忽略了。在这里,我们使用远红外光谱法来监测四种丰富的非生物活性蛋白的结构,分别是大豆蛋白分离物(SPI),豌豆蛋白分离物(PPI)和两种类型的丝素蛋白(SF),即家蚕和野生蚕丝蛋白。ther药。与先前研究的其他一些生物活性球蛋白(溶菌酶,肌红蛋白,血红蛋白等)一致,两种球形蛋白SPI和PPI导致宽和弱的远红外波段(介于50和700 cm -1之间)。),通常只有随机的氨基酸序列。有趣的是,这两个SF的特征是由高度重复的基序组成的结构显示出几个尖锐的远红外特征吸收峰。而且,这些特征峰中的一些(例如,在桑蚕中的260和428cm -1处的峰,以及在百日草中的245和448cm -1处的峰)对构象变化敏感。因此,它们可以直接用于监测SF中的构象转变。此外,由于SF吸收带明显不同于球形蛋白,并且不同的SF甚至显示出不同的吸收带,因此可以使用远红外光谱法来区分和确定蛋白质混合物中的特定SF成分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号