Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-04-04 , DOI: 10.1016/j.tetlet.2018.03.063 Ryota Kajihara , Shingo Harada , Jun Ueda , Tetsuhiro Nemoto
|
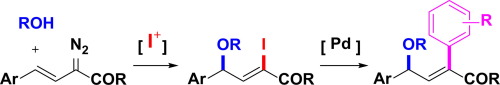
Diazo compounds are frequently used as precursors of metal carbenoids and act as soft nucleophiles even without the use of metal catalysts. The resulting diazonium species may also be trapped by various nucleophiles. The introduction of an iodine functionality applicable for the coupling reaction into an alkenyl diazo compound, however, has not been reported. We developed iodoalkoxylation reactions of alkenyl diazoacetates using an electrophilic iodinating reagent and oxygen nucleophile. This catalyst-free multicomponent reaction proceeded regioselectively, furnishing trisubstituted vinyl iodides in 31%–71% yield. The synthesized iodoalkenes were converted into the corresponding olefins with various functionalities in good yield via Suzuki-Miyaura coupling reactions with arylboronic acids under palladium catalysis to demonstrate the synthetic utility of the developed reaction sequence.
中文翻译:

使用由烯基重氮乙酸酯的亲电碘化引发的多组分反应合成功能化碘代烯烃
重氮化合物经常用作金属类胡萝卜素的前体,即使不使用金属催化剂也可作为柔软的亲核试剂。所得重氮鎓物质也可能被各种亲核试剂捕获。然而,尚未报道将适用于偶联反应的碘官能团引入烯基重氮化合物中。我们使用亲电子碘化试剂和氧亲核试剂开发了重氮烯基乙酸酯的碘烷氧基化反应。这种无催化剂的多组分反应可以区域选择性地进行,提供了31%–71%收率的三取代乙烯基碘。











































 京公网安备 11010802027423号
京公网安备 11010802027423号