Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2018-04-04 , DOI: 10.1016/j.micromeso.2018.04.007 Firas A. Abdul Kareem , A.M. Shariff , Sami Ullah , Nurhayati Mellon , L.K. Keong
|
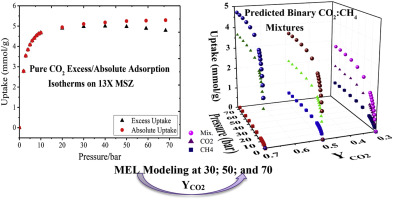
The growth in energy demand for natural gas has led to the exploration of sub-quality natural gas reserves with high CO2 content up to 80% at the offshore condition with temperature and pressure approximately 50 °C and 70 bar. In this work, a gravimetric technique is used to study CO2 and CH4 adsorptions on 13× zeolites at 50 °C and up to 70 bar pressure as an adequate range for offshore operations. 13× zeolite shows high CO2 adsorption capacity with 5.226 mmol/g at 50 °C compared to 4.29 mmol/g at 70 °C. The same trend is noticed for CH4 adsorption on both temperatures. Four equilibrium isotherm models are used to analyze the adsorption data i.e. Langmuir, Freundlich, Toth, and Sips. Virial isotherm model is applied on the experimental data to illustrate the isosteric heat of adsorption and it shows an excellent agreement with R2 = 0.998 for 13× MSZ. Henry's law constant is estimated utilizing Virial coefficients, which shows higher molar selectivity ratio for CO2 on 13× with α ˜ 3.957 at 50 °C as compared to the α ˜ 3.736 at 70 °C. Extended Langmuir (EL) Model and Multisite Langmuir (MSL) models are applied for 30:70, 50:50, and 70:30 CO2:CH4 binary mixtures. The outcomes of MSL exhibit high agreement with the quadrupole and polarizability of the single component and the mixtures. The kinetic rate constant is estimated according to the applied LDF model at higher operational regions. The 13× MSZ shows feasibly good isosteric heat of adsorption, which might refer to the large surface area and pore volume that can accommodate CO2 at higher speed and quantity at offshore conditions.
中文翻译:

纯的和预测的二元(CO 2:CH 4)混合物在13X沸石上的吸附:海上条件下的平衡和动力学性质
天然气能源需求的增长已导致在海上条件下,温度和压力约为50°C和70 bar的情况下,勘探高品质CO 2含量高达80%的次优天然气储量。在这项工作中,使用重量分析技术研究了在50°C和最高70 bar的压力下,CO 2和CH 4在13x沸石上的吸附量,这是海上作业的合适范围。13x沸石在50°C时具有5.226 mmol / g的高CO 2吸附能力,而在70°C时为4.29 mmol / g。CH 4的趋势相同在两个温度下均吸附。四个平衡等温线模型用于分析吸附数据,即Langmuir,Freundlich,Toth和Sips。在实验数据上采用病毒等温线模型来说明吸附的等规热,对于13x MSZ ,它与R 2 = 0.998表现出极好的一致性。亨利定律常数是利用Virial系数估算的,与在70°C的α〜3.736相比,CO 2在13x上的α〜3.957在50°C下具有更高的摩尔选择性。扩展Langmuir(EL)模型和多站点Langmuir(MSL)模型适用于30:70、50:50和70:30 CO 2:CH 4二元混合物。MSL的结果与单组分和混合物的四极杆和极化率高度吻合。动力学速率常数是根据在较高工作区域应用的LDF模型估算的。13倍MSZ显示出切实可行的等规吸附热,这可能是指大表面积和孔体积可以在海上条件下以较高的速度和量容纳CO 2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号