Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2018-04-04 , DOI: 10.1016/j.molliq.2018.04.009 Danfeng Shao , Zehui Yang , Guoquan Zhou , Jingjing Chen , Shiyun Zheng , Xin Lv , Rongrong Li
|
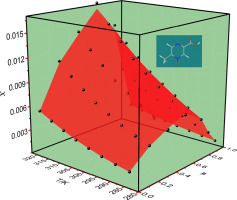
For the solubility of poorly soluble acipimox to be improved, study on cosolvent selection has often been shown to be advantageous. The solubility values of acipimox in four important solvents for pharmaceuticals investigations: water, methanol, acetone, acetonitrile and their aqueous solutions have been measured at atmospheric pressure using isothermal saturation method at temperatures ranging from 283.15 to 318.15 K, ΔT = 5 K and in the mass concentration range from 0 to 1. The result showed that the solubility increased to a maximum value (at 80% mass fraction in methanol aqueous solution and acetone aqueous solution, while 60% for acetonitrile aqueous solution) and then decreased with further increase of cosolvent mass concentration. The maximum mole fraction solubility of acipimox was observed in acetonitrile aqueous (1.687 × 10−2 at 318.15 K), followed by that in acetone aqueous (1.258 × 10−2 at 318.15 K), methanol aqueous (8.370 × 10−3 at 318.15 K), The Jouyban-Acree model, van't Hoff-Jouyban-Acree model, Apelblat-Jouyban-Acree model and Ma model were applied to simulate acipimox solubility in the binary mixture compositions at various temperatures. The relative average deviation (RAD) was used as an accuracy criterion. The obtained overall RAD for back-calculated of acipimox in studied mixtures varied from 1.24% to 4.07%. Apparent thermodynamic analysis indicated that the dissolution was an endothermic, spontaneous and entropy driven process. Understanding acipimox solubility and thermodynamic properties is an important aspect of drug development. In particular, the solubility data of acipimox obtained has a major effect on its purification, recrystallization and formulation development in industrial.
中文翻译:

通过助溶剂提高阿西莫司的溶解度和溶剂化过程中热力学性质的研究
为了改善难溶性阿西莫司的溶解性,研究助溶剂的选择常常被证明是有利的。在四个重要溶剂药品调查阿昔莫司的溶解度值:水,甲醇,丙酮,乙腈和它们的水溶液都在大气压下使用等温饱和方法在温度范围从283.15到318.15 K,Δ被测量Ť = 5 K,质量浓度范围为0到1。结果显示,溶解度增加到最大值(在甲醇水溶液和丙酮水溶液中为80%的质量分数,而在乙腈水溶液中为60%的溶解度),然后随着助溶剂质量浓度的进一步增加而降低。在乙腈水溶液(318.15 K,1.687×10 -2)中观察到阿西莫司的最大摩尔分数溶解度,其次在丙酮水溶液(318.15 K,1.258×10 -2),甲醇水溶液(8.370×10 -3)中观察到最大摩尔分数溶解度在318.15 K),应用Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型,Apelblat-Jouyban-Acree模型和Ma模型来模拟acipimox在不同温度下在二元混合物组成中的溶解度。相对平均偏差(RAD)用作准确性标准。用于研究混合物中阿昔莫昔的反算所获得的总RAD范围从1.24%到4.07%。明显的热力学分析表明溶出是一个吸热,自发和熵驱动的过程。了解acipimox的溶解度和热力学性质是药物开发的重要方面。特别地,所获得的阿西莫克斯的溶解度数据对其在工业中的纯化,重结晶和制剂开发具有重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号