Cellular Signalling ( IF 4.4 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.cellsig.2018.01.008 Joana Lérias , Madalena Pinto , Roberta Benedetto , Rainer Schreiber , Margarida Amaral , Massimo Aureli , Karl Kunzelmann
|
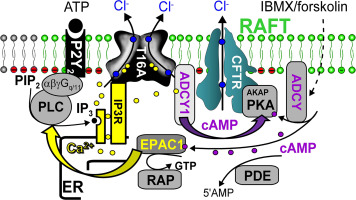
Airway epithelial cells express both Ca2+ activated TMEM16A/ANO1 and cAMP activated CFTR anion channels. Previous work suggested a significant crosstalk of intracellular Ca2+ and cAMP signaling pathways, leading to activation of both chloride channels. We demonstrate that in airway epithelial cells, stimulation of purinergic or muscarinic G-protein coupled receptors (GPCRs) activates TMEM16A and CFTR. Additional expression of Gq/11 and phospholipase C coupled GPCRs strongly enhanced the crosstalk between Ca2+- and cAMP-dependent signaling. Knockdown of endogenous GRCRs attenuated crosstalk and functional coupling between TMEM16A and CFTR. The number of receptors did not affect expression or membrane localization of TMEM16A or CFTR, but controlled assembly of the local signalosome. GPCRs translocate Ca2+-sensitive adenylate cyclase type 1 (ADCY1) and exchange protein directly activated by cAMP (EPAC1) to particular plasma membrane domains containing GPCRs, CFTR and TMEM16A, thereby producing compartmentalized Ca2+ and cAMP signals and significant crosstalk. While biosynthesis and membrane trafficking of CFTR requires a functional Golgi apparatus, maturation and membrane trafficking of TMEM16A may occur independent of the Golgi. Because Ca2+ activated TMEM16A currents are only transient, continuous Cl− secretion by airway epithelial cells requires CFTR. The present data also explain why receptor-dependent activation of TMEM16A is more efficient than direct stimulation by Ca2+.
中文翻译:

通过EPAC1和ADCY1进行CFTR和TMEM16A(ANO1)的分区串扰
气道上皮细胞表达Ca 2+激活的TMEM16A / ANO1和cAMP激活的CFTR阴离子通道。先前的工作表明细胞内Ca 2+和cAMP信号通路之间存在显着串扰,导致两个氯离子通道均被激活。我们证明,在气道上皮细胞中,嘌呤能或毒蕈碱性G蛋白偶联受体(GPCRs)的刺激会激活TMEM16A和CFTR。G q / 11和磷脂酶C偶联GPCR的额外表达强烈增强了Ca 2+之间的串扰-和依赖cAMP的信号传导。内源性GRCR的组合降低了TMEM16A和CFTR之间的串扰和功能耦合。受体的数量不影响TMEM16A或CFTR的表达或膜定位,但可以控制局部信号小体的组装。GPCR将Ca 2+敏感的1型腺苷酸环化酶(ADCY1)易位,并将由cAMP(EPAC1)直接激活的蛋白质交换到包含GPCR,CFTR和TMEM16A的特定质膜结构域,从而产生分隔的Ca 2+和cAMP信号以及明显的串扰。尽管CFTR的生物合成和膜运输需要功能性的高尔基体,但TMEM16A的成熟和膜运输可能独立于高尔基体。因为Ca 2+激活TMEM16A电流只是短暂的,连续氯-分泌由气道上皮细胞,需要CFTR。本数据还解释了为什么TMEM16A的受体依赖性激活比Ca 2+的直接刺激更有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号