当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of Hexadentate Bispidine Ligands as Chelators for 64 CuII PET Imaging.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-04-20 , DOI: 10.1002/cplu.201800110 Peter Comba 1 , Miriam Starke 1 , Hubert Wadepohl 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-04-20 , DOI: 10.1002/cplu.201800110 Peter Comba 1 , Miriam Starke 1 , Hubert Wadepohl 1
Affiliation
|
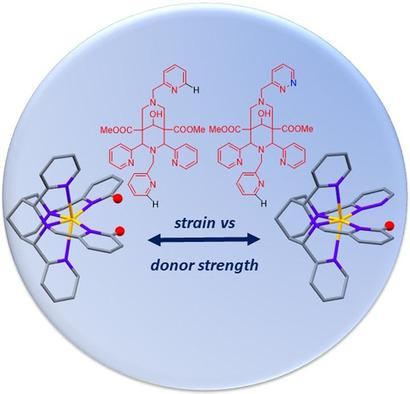
The coordination chemistry of the hexadentate bispidine ligand L1 with three pyridine, one pyridazine, and two tertiary amine donors with CuII and ZnII is reported. The substitution of one of the two cis-disposed pyridine donors in-plane with the two tertiary amines of the bispidine backbone by a pyridazine group was predicted to substantially reduce distortion from planarity of the tetradentate subunit and therefore was assumed to lead to higher CuII complex stability. The X-ray single-crystal structures of the ZnII and CuII complexes, which are in excellent agreement with the DFT- and MM-optimized structures, confirmed this prediction but indicated that deviation from planarity is appreciable. This also emerges from solution spectroscopy (UV/Vis/NIR and EPR of the CuII complex), which indicated that the in-plane ligand field is only slightly increased. The geometric parameters together with the lower basicity of pyridazine versus pyridine (pKa =2.33 vs. 5.23) are also in agreement with an only slightly more negative redox potential of the CuII/I couple (Eo =-780 vs. -760 mV, MeCN, vs. Fc/Fc+ ), and the potentiometrically determined CuII stability constant with L1 is slightly lower than that with the parent ligand L2 (log K=12.7 vs. 14.5). Therefore, modification of the donor groups is a more promising approach to increasing the stabilities of these complexes and yields 64 CuII chelators that outperform known hexadentate bispidine ligands.
中文翻译:

用于64 CuII PET成像的作为螯合剂的六齿联苯吡啶配体的优化。
报道了六齿联吡啶配体L1与三个吡啶,一个哒嗪和两个叔胺供体与CuII和ZnII的配位化学。平面上两个联吡啶骨架的两个叔胺在平面上取代了两个顺式配置的吡啶供体之一,据推测可大大降低四齿亚基的平面度造成的畸变,因此被认为会导致更高的CuII络合物稳定。ZnII和CuII配合物的X射线单晶结构与DFT和MM优化结构高度吻合,证实了这一预测,但表明与平面度的偏差是可观的。这也来自溶液光谱法(CuII配合物的UV / Vis / NIR和EPR),这表明面内配体场仅略有增加。几何参数以及哒嗪对吡啶的较低碱度(pKa = 2.33对5.23)也与CuII / I对的负氧化还原电势稍高(Eo = -780对-760 mV,MeCN)一致,相对于Fc / Fc +),通过电位计确定的LII的CuII稳定性常数略低于亲本配体L2的CuII稳定性常数(log K = 12.7 vs. 14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。23)也与CuII / I对的负氧化还原电势稍高一些(Eo = -780对-760 mV,MeCN对Fc / Fc +),并且用L1电位确定的CuII稳定性常数为略低于亲本配体L2的水平(log K = 12.7对14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。23)也与CuII / I对的负氧化还原电势稍高一些(Eo = -780对-760 mV,MeCN对Fc / Fc +),并且用L1电位确定的CuII稳定性常数为略低于亲本配体L2的水平(log K = 12.7对14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。
更新日期:2018-04-20
中文翻译:

用于64 CuII PET成像的作为螯合剂的六齿联苯吡啶配体的优化。
报道了六齿联吡啶配体L1与三个吡啶,一个哒嗪和两个叔胺供体与CuII和ZnII的配位化学。平面上两个联吡啶骨架的两个叔胺在平面上取代了两个顺式配置的吡啶供体之一,据推测可大大降低四齿亚基的平面度造成的畸变,因此被认为会导致更高的CuII络合物稳定。ZnII和CuII配合物的X射线单晶结构与DFT和MM优化结构高度吻合,证实了这一预测,但表明与平面度的偏差是可观的。这也来自溶液光谱法(CuII配合物的UV / Vis / NIR和EPR),这表明面内配体场仅略有增加。几何参数以及哒嗪对吡啶的较低碱度(pKa = 2.33对5.23)也与CuII / I对的负氧化还原电势稍高(Eo = -780对-760 mV,MeCN)一致,相对于Fc / Fc +),通过电位计确定的LII的CuII稳定性常数略低于亲本配体L2的CuII稳定性常数(log K = 12.7 vs. 14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。23)也与CuII / I对的负氧化还原电势稍高一些(Eo = -780对-760 mV,MeCN对Fc / Fc +),并且用L1电位确定的CuII稳定性常数为略低于亲本配体L2的水平(log K = 12.7对14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。23)也与CuII / I对的负氧化还原电势稍高一些(Eo = -780对-760 mV,MeCN对Fc / Fc +),并且用L1电位确定的CuII稳定性常数为略低于亲本配体L2的水平(log K = 12.7对14.5)。因此,对供体基团的修饰是增加这些复合物稳定性的更有前途的方法,可产生64种CuII螯合剂,其性能优于已知的六齿双吡啶配体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号