Biochimie ( IF 3.3 ) Pub Date : 2018-04-03 , DOI: 10.1016/j.biochi.2018.03.014 Shikha Sharma , Mushtaq Ahmed , Yusuf Akhter
|
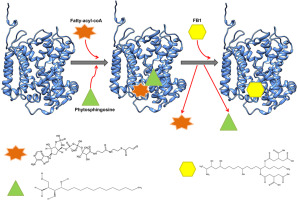
Fumonisin B1 toxin (FB1) is a well-known competitive inhibitor of ceramide synthase (CS) in yeast. However, FB1 is unable to obstruct CS from Trichoderma spp., which are well-known biocontrol agents. To explore the contrasting binding modes, a comparative structural analysis of complexes of FB1 with these two CS proteins was carried out. Formation of activation loop on the binding of substrates with the CS from yeast was observed but when inhibitor interacted with the activation loop, it transformed into helix leading to the potentially inactivated state of the enzyme. In yeast homologue of the enzyme, the inhibitor and substrates compete for the same binding site. Whereas, in the CS protein from Trichoderma guizhouense, no such competition for substrate binding site was observed and the binding pocket of the enzyme could easily accommodate FB1 molecule along with the two interacting native substrates, which may lead to the successful catalysis.
中文翻译:

选择性鞘脂途径抑制机制对伏马菌毒素与易感生物体神经酰胺合成酶结合的影响及抗性物种的生存机制的启示
伏马菌素B1毒素(FB1)是酵母中神经酰胺合酶(CS)的著名竞争性抑制剂。但是,FB1无法阻止木霉属的木霉菌CS ,这是众所周知的生物防治剂。为了探索相反的结合方式,对FB1与这两种CS蛋白的复合物进行了比较结构分析。观察到在底物与来自酵母的CS结合的底物上形成了活化环,但是当抑制剂与活化环相互作用时,它转化为螺旋体,导致了酶的潜在失活状态。在酶的酵母同源物中,抑制剂和底物竞争相同的结合位点。而贵州木霉的CS蛋白中,没有观察到这样的对底物结合位点的竞争,并且该酶的结合口袋可以容易地将FB1分子与两个相互作用的天然底物一起容纳,这可能导致成功的催化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号