当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein–Protein Interactions in the Molecular Chaperone Network
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1021/acs.accounts.8b00036 Rebecca Freilich 1 , Taylor Arhar 1 , Jennifer L. Abrams 1 , Jason E. Gestwicki 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1021/acs.accounts.8b00036 Rebecca Freilich 1 , Taylor Arhar 1 , Jennifer L. Abrams 1 , Jason E. Gestwicki 1
Affiliation
|
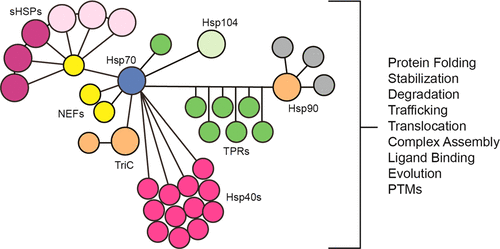
Molecular chaperones play a central role in protein homeostasis (a.k.a. proteostasis) by balancing protein folding, quality control, and turnover. To perform these diverse tasks, chaperones need the malleability to bind nearly any “client” protein and the fidelity to detect when it is misfolded. Remarkably, these activities are carried out by only ∼180 dedicated chaperones in humans. How do a relatively small number of chaperones maintain cellular and organismal proteostasis for an entire proteome? Furthermore, once a chaperone binds a client, how does it “decide” what to do with it? One clue comes from observations that individual chaperones engage in protein–protein interactions (PPIs)—both with each other and with their clients. These physical links coordinate multiple chaperones into organized, functional complexes and facilitate the “handoff” of clients between them. PPIs also link chaperones and their clients to other cellular pathways, such as those that mediate trafficking (e.g., cytoskeleton) and degradation (e.g., proteasome). The PPIs of the chaperone network have a wide range of affinity values (nanomolar to micromolar) and involve many distinct types of domain modules, such as J domains, zinc fingers, and tetratricopeptide repeats. Many of these motifs have the same binding surfaces on shared partners, such that members of one chaperone class often compete for the same interactions. Somehow, this collection of PPIs draws together chaperone families and creates multiprotein subnetworks that are able to make the “decisions” of protein quality control. The key to understanding chaperone-mediated proteostasis might be to understand how PPIs are regulated.
中文翻译:

分子伴侣网络中蛋白质与蛋白质的相互作用
分子伴侣通过平衡蛋白质折叠,质量控制和周转而在蛋白质稳态(aka蛋白质稳态)中发挥重要作用。为了执行这些多样化的任务,分子伴侣需要具有可延展性才能结合几乎所有“客户”蛋白质,并需要保真度以检测其折叠错误时的状态。值得注意的是,这些活动仅由约180个人类专用伴侣进行。相对较少的伴侣分子如何维持整个蛋白质组的细胞和生物蛋白质溶解?此外,一旦伴侣绑定了客户,它如何“决定”如何处理客户?一个线索来自观察到,各个伴侣之间相互之间以及与客户之间都参与了蛋白质间相互作用(PPI)。这些物理联系将多个分子伴侣协调在一起,功能复合体,并促进客户之间的“交接”。PPI还将伴侣蛋白及其客户与其他细胞途径联系起来,例如介导运输(例如细胞骨架)和降解(例如蛋白酶体)的那些细胞途径。伴侣网络的PPI具有广泛的亲和力值(纳摩尔至微摩尔),并且涉及许多不同类型的域模块,例如J域,锌指和四肽重复序列。这些主题中有许多在共享伴侣上具有相同的结合表面,因此一个伴侣类别的成员经常竞争相同的相互作用。不知何故,这些PPI集合了分子伴侣家族,并创建了多蛋白质子网络,这些网络可以做出蛋白质质量控制的“决定”。
更新日期:2018-04-03
中文翻译:

分子伴侣网络中蛋白质与蛋白质的相互作用
分子伴侣通过平衡蛋白质折叠,质量控制和周转而在蛋白质稳态(aka蛋白质稳态)中发挥重要作用。为了执行这些多样化的任务,分子伴侣需要具有可延展性才能结合几乎所有“客户”蛋白质,并需要保真度以检测其折叠错误时的状态。值得注意的是,这些活动仅由约180个人类专用伴侣进行。相对较少的伴侣分子如何维持整个蛋白质组的细胞和生物蛋白质溶解?此外,一旦伴侣绑定了客户,它如何“决定”如何处理客户?一个线索来自观察到,各个伴侣之间相互之间以及与客户之间都参与了蛋白质间相互作用(PPI)。这些物理联系将多个分子伴侣协调在一起,功能复合体,并促进客户之间的“交接”。PPI还将伴侣蛋白及其客户与其他细胞途径联系起来,例如介导运输(例如细胞骨架)和降解(例如蛋白酶体)的那些细胞途径。伴侣网络的PPI具有广泛的亲和力值(纳摩尔至微摩尔),并且涉及许多不同类型的域模块,例如J域,锌指和四肽重复序列。这些主题中有许多在共享伴侣上具有相同的结合表面,因此一个伴侣类别的成员经常竞争相同的相互作用。不知何故,这些PPI集合了分子伴侣家族,并创建了多蛋白质子网络,这些网络可以做出蛋白质质量控制的“决定”。











































 京公网安备 11010802027423号
京公网安备 11010802027423号