当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00126 P. Jack Hoopes 1, 2, 3 , Robert J. Wagner 1 , Kayla Duval 2 , Kevin Kang 2 , David J. Gladstone 1, 2, 3 , Karen L Moodie 1 , Margaret Crary-Burney 1 , Hugo Ariaspulido 1 , Frank A. Veliz 4 , Nicole F. Steinmetz 4 , Steven N. Fiering 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00126 P. Jack Hoopes 1, 2, 3 , Robert J. Wagner 1 , Kayla Duval 2 , Kevin Kang 2 , David J. Gladstone 1, 2, 3 , Karen L Moodie 1 , Margaret Crary-Burney 1 , Hugo Ariaspulido 1 , Frank A. Veliz 4 , Nicole F. Steinmetz 4 , Steven N. Fiering 1
Affiliation
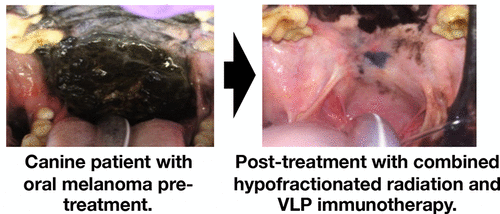
|
The presence and benefit of a radiation therapy-associated immune reaction is of great interest as the overall interest in cancer immunotherapy expands. The pathological assessment of irradiated tumors rarely demonstrates consistent immune or inflammatory response. More recent information, primarily associated with the “abscopal effect”, suggests a subtle radiation-based systemic immune response may be more common and have more therapeutic potential than previously believed. However, to be of consistent value, the immune stimulatory potential of radiation therapy (RT) will clearly need to be supported by combination with other immunotherapy efforts. In this study, using a spontaneous canine oral melanoma model, we have assessed the efficacy and tumor immunopathology of two nanotechnology-based immune adjuvants combined with RT. The immune adjuvants were administered intratumorally, in an approach termed “in situ vaccination”, that puts immunostimulatory reagents into a recognized tumor and utilizes the endogenous antigens in the tumor as the antigens in the antigen/adjuvant combination that constitutes a vaccine. The radiation treatment consisted of a local 6 × 6 Gy tumor regimen given over a 12 day period. The immune adjuvants were a plant-based virus-like nanoparticle (VLP) and a 110 nm diameter magnetic iron oxide nanoparticle (mNPH) that was activated with an alternating magnetic field (AMF) to produce moderate heat (43 °C/60 min). The RT was used alone or combined with one or both adjuvants. The VLP (4 × 200 μg) and mNPH (2 × 7.5 mg/gram tumor) were delivered intratumorally respectively during the RT regimen. All patients received a diagnostic biopsy and CT-based 3-D radiation treatment plan prior to initiating therapy. Patients were assessed clinically 14–21 days post-treatment, monthly for 3 months following treatment, and bimonthly, thereafter. Immunohistopathologic assessment of the tumors was performed before and 14–21 days following treatment. Results suggest that addition of VLPs and/or mNPH to a hypofractionated radiation regimen increases the immune cell infiltration in the tumor, extends the tumor control interval, and has important systemic therapeutic potential.
中文翻译:

基于纳米技术的免疫治疗和放射治疗犬口腔黑素瘤
随着对癌症免疫疗法的整体兴趣的扩大,与放射疗法相关的免疫反应的存在和益处受到极大关注。放射肿瘤的病理评估很少显示出一致的免疫或炎症反应。主要与“绝对效应”相关的最新信息表明,基于微弱辐射的全身免疫应答可能比以前认为的更为普遍并且具有更大的治疗潜力。但是,要具有一致的价值,显然需要结合其他免疫疗法来支持放射疗法(RT)的免疫刺激潜力。在这项研究中,使用自发的犬类口腔黑色素瘤模型,我们评估了两种基于纳米技术的免疫佐剂联合RT的疗效和肿瘤免疫病理学。免疫佐剂以称为“原位疫苗接种”的方法在肿瘤内施用,该方法将免疫刺激剂放入公认的肿瘤中,并利用肿瘤中的内源性抗原作为构成疫苗的抗原/佐剂组合中的抗原。放射治疗包括在12天内进行的局部6×6 Gy肿瘤治疗方案。免疫佐剂是基于植物的病毒样纳米颗粒(VLP)和直径110 nm的磁性氧化铁纳米颗粒(mNPH),其被交变磁场(AMF)激活以产生中等热量(43°C / 60分钟) 。RT单独使用或与一种或两种佐剂结合使用。在RT方案中,分别在肿瘤内递送VLP(4×200μg)和mNPH(2×7.5 mg / g肿瘤)。在开始治疗之前,所有患者均接受了诊断性活检和基于CT的3-D放射治疗计划。患者在治疗后14-21天进行临床评估,治疗后3个月每月一次,此后每2个月进行一次临床评估。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。
更新日期:2018-04-03
中文翻译:

基于纳米技术的免疫治疗和放射治疗犬口腔黑素瘤
随着对癌症免疫疗法的整体兴趣的扩大,与放射疗法相关的免疫反应的存在和益处受到极大关注。放射肿瘤的病理评估很少显示出一致的免疫或炎症反应。主要与“绝对效应”相关的最新信息表明,基于微弱辐射的全身免疫应答可能比以前认为的更为普遍并且具有更大的治疗潜力。但是,要具有一致的价值,显然需要结合其他免疫疗法来支持放射疗法(RT)的免疫刺激潜力。在这项研究中,使用自发的犬类口腔黑色素瘤模型,我们评估了两种基于纳米技术的免疫佐剂联合RT的疗效和肿瘤免疫病理学。免疫佐剂以称为“原位疫苗接种”的方法在肿瘤内施用,该方法将免疫刺激剂放入公认的肿瘤中,并利用肿瘤中的内源性抗原作为构成疫苗的抗原/佐剂组合中的抗原。放射治疗包括在12天内进行的局部6×6 Gy肿瘤治疗方案。免疫佐剂是基于植物的病毒样纳米颗粒(VLP)和直径110 nm的磁性氧化铁纳米颗粒(mNPH),其被交变磁场(AMF)激活以产生中等热量(43°C / 60分钟) 。RT单独使用或与一种或两种佐剂结合使用。在RT方案中,分别在肿瘤内递送VLP(4×200μg)和mNPH(2×7.5 mg / g肿瘤)。在开始治疗之前,所有患者均接受了诊断性活检和基于CT的3-D放射治疗计划。患者在治疗后14-21天进行临床评估,治疗后3个月每月一次,此后每2个月进行一次临床评估。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。在治疗前和治疗后14-21天进行了肿瘤的免疫组织病理学评估。结果表明,将VLP和/或mNPH添加到低级放疗方案中会增加肿瘤中免疫细胞的浸润,延长肿瘤的控制间隔,并具有重要的全身治疗潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号