Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2018-04-03 , DOI: 10.1016/j.molliq.2018.03.093 Qing Liao , Wang Pan , Dongsheng Zou , Runpu Shen , Guodong Sheng , Xue Li , Yuling Zhu , Lijia Dong , Abdullah M. Asiri , Khalid A. Alamry , Wensheng Linghu
|
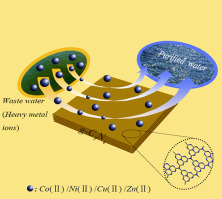
Graphitic-C3N4 (g-C3N4) nanosheets were prepared by a simple thermal and ultrasonic method. The crystal structure, surface functional groups and morphology of g-C3N4 were characterized by XRD, FITR, SEM, TEM and XPS. The scavenging of heavy metals on g-C3N4 was investigated at environmental relevant concentrations by using batch experiments. The results indicated that g-C3N4 showed high adsorption capacities of 137.4 mg/g for Co(II), 136.9 mg/g for Ni(II), 134.1 mg/g for Cu(II), and 138.0 mg/g for Zn(II), respectively. The Temkin, Freundlich and Langmuir models were used to simulate the adsorption isotherms of heavy metals, demonstrating that the adsorption fitted well with Langmuir model. The ionic strength exhibited little effect on the adsorption of heavy metals on g-C3N4, indicating that the adsorption mechanism of Co(II), Ni(II), Cu(II), Zn(II) on g-C3N4 was mainly dominated by inner-sphere complexation. The thermodynamic data calculated from adsorption isotherms suggested that the adsorption of heavy metals on g-C3N4 was an endothermic and spontaneous process. These results demonstrated that the g-C3N4 was a potential material for the scavenging of heavy metals in water.
中文翻译:

使用gC 3 N 4纳米片在环境相关浓度下高效清除重金属
通过简单的热和超声方法制备了Graphictic-C 3 N 4(gC 3 N 4)纳米片。用XRD,FITR,SEM,TEM和XPS对gC 3 N 4的晶体结构,表面官能团和形貌进行了表征。使用分批实验研究了在环境相关浓度下gC 3 N 4上重金属的清除作用。结果表明,gC 3 N 4分别显示出对Co(II)的137.4 mg / g,对Ni(II)136.9 mg / g,对Cu(II)134.1 mg / g和对Zn(II)138.0 mg / g的高吸附能力。用Temkin,Freundlich和Langmuir模型模拟重金属的吸附等温线,表明吸附与Langmuir模型拟合得很好。离子强度对重金属在gC 3 N 4上的吸附几乎没有影响,表明Co(II),Ni(II),Cu(II),Zn(II)在gC 3 N 4上的吸附机理主要是以内球络合为主。由吸附等温线计算的热力学数据表明,重金属在gC 3 N 4上的吸附是一个吸热和自发的过程。这些结果表明,gC 3 N 4是清除水中重金属的潜在材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号