Bioelectrochemistry ( IF 4.8 ) Pub Date : 2018-03-21 , DOI: 10.1016/j.bioelechem.2018.03.011 Janja Dermol-Černe , Damijan Miklavčič , Matej Reberšek , Primož Mekuč , Sylvia M. Bardet , Ryan Burke , Delia Arnaud-Cormos , Philippe Leveque , Rodney O'Connor
|
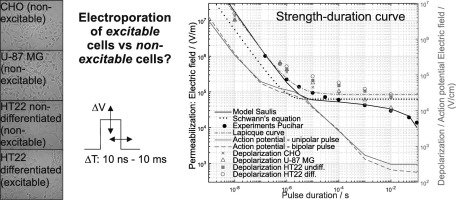
In electroporation-based medical treatments, excitable tissues are treated, either intentionally (irreversible electroporation of brain cancer, gene electrotransfer or ablation of the heart muscle, gene electrotransfer of skeletal muscles), or unintentionally (excitable tissues near the target area). We investigated how excitable and non-excitable cells respond to electric pulses, and if electroporation could be an effective treatment of the tumours of the central nervous system. For three non-excitable and one excitable cell line, we determined a strength-duration curve for a single pulse of 10 ns–10 ms. The threshold for depolarization decreased with longer pulses and was higher for excitable cells. We modelled the response with the Lapicque curve and the Hodgkin-Huxley model. At 1 μs a plateau of excitability was reached which could explain why high-frequency irreversible electroporation (H-FIRE) electroporates but does not excite cells. We exposed cells to standard electrochemotherapy parameters (8 × 100 μs pulses, 1 Hz, different voltages). Cells behaved similarly which indicates that electroporation most probably occurs at the level of lipid bilayer, independently of the voltage-gated channels. These results could be used for optimization of electric pulses to achieve maximal permeabilization and minimal excitation/pain sensation. In the future, it should be established whether the in vitro depolarization correlates to nerve/muscle stimulation and pain sensation in vivo.
中文翻译:

不同兴奋性细胞系中电脉冲引起的质膜去极化和通透性
在基于电穿孔的医学治疗中,对可兴奋性组织进行有意地治疗(脑癌的不可逆电穿孔,心肌的基因电转移或消融,骨骼肌的基因电转移)或无意地(目标区域附近的兴奋性组织)进行治疗。我们研究了兴奋性和非兴奋性细胞如何响应电脉冲,以及电穿孔是否可以有效治疗中枢神经系统肿瘤。对于三种非兴奋性细胞和一种兴奋性细胞系,我们确定了10 ns–10 ms单个脉冲的强度-持续时间曲线。去极化的阈值随着更长的脉冲而降低,而对于可激发细胞则更高。我们使用Lapicque曲线和Hodgkin-Huxley模型对响应进行建模。在1μs时达到了兴奋性的平稳状态,这可以解释为什么高频不可逆电穿孔(H-FIRE)会电穿孔但不会刺激细胞。我们将细胞暴露于标准的电化学疗法参数(8×100μs脉冲,1 Hz,不同电压)。细胞的行为相似,这表明电穿孔最有可能发生在脂质双层的水平,与电压门控通道无关。这些结果可用于优化电脉冲,以实现最大的通透性和最小的激励/疼痛感。将来,应该确定体外去极化是否与体内神经/肌肉刺激和疼痛感相关。我们将细胞暴露于标准的电化学疗法参数(8×100μs脉冲,1 Hz,不同电压)。细胞的行为相似,这表明电穿孔最有可能发生在脂质双层的水平,与电压门控通道无关。这些结果可用于优化电脉冲,以实现最大的通透性和最小的激励/疼痛感。将来,应该确定体外去极化是否与体内神经/肌肉刺激和疼痛感相关。我们将细胞暴露于标准的电化学疗法参数(8×100μs脉冲,1 Hz,不同电压)。细胞的行为相似,这表明电穿孔最有可能发生在脂质双层的水平,与电压门控通道无关。这些结果可用于优化电脉冲,以实现最大的通透性和最小的激励/疼痛感。将来,应该确定体外去极化是否与体内神经/肌肉刺激和疼痛感相关。这些结果可用于优化电脉冲,以实现最大的通透性和最小的激励/疼痛感。将来,应该确定体外去极化是否与体内神经/肌肉刺激和疼痛感相关。这些结果可用于优化电脉冲,以实现最大的通透性和最小的激励/疼痛感。将来,应该确定体外去极化是否与体内神经/肌肉刺激和疼痛感相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号