当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(i)-catalyzed cycloisomerization of ortho-(alkynyl) styrenes: DFT analysis of the crucial role of SbF6− in the elimination of protons†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c8cy00367j Ran Fang 1, 2, 3, 4, 5 , Lin Zhou 1, 2, 3, 4, 5 , Peng-Cheng Tu 1, 2, 3, 4, 5 , Lizi Yang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c8cy00367j Ran Fang 1, 2, 3, 4, 5 , Lin Zhou 1, 2, 3, 4, 5 , Peng-Cheng Tu 1, 2, 3, 4, 5 , Lizi Yang 1, 2, 3, 4, 5
Affiliation
|
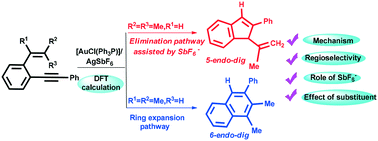
This study reports the first theoretical evidence for the crucial role played by SbF6− in the determination of the regioselectivity in the gold-catalyzed cycloisomerization of ortho-(phenylethynyl) styrenes. According to our calculations, irrespective of using α-substituted or β,β-disubstituted o-(phenylethynyl) styrene, a novel and unusual reaction pathway for elimination of protons involving a four-center-three-electron intermediate, instead of the generation of HSbF6, is observed. On account of the electronic effect, the ring expansion pathway is more favorable than the elimination pathway for α-substituted o-(phenylethynyl) styrene. By using β,β-disubstituted o-(phenylethynyl) styrene, an elimination pathway involving the four-center-three-electron intermediate affords the indenyl derivative. Based on our calculation results and experimental facts, the regioselectivity for the title reaction is mainly controlled by electronic effects rather than steric effects. The theoretical results not only well rationalize the experimental observations but also provide insights into the details of the reaction.
中文翻译:

黄金(我)的催化的环异构邻- (炔基)苯乙烯:的SBF的至关重要的作用DFT分析6 -在消除质子†
这项研究报告通过的SbF发挥的重要作用的第一个理论依据6 -在黄金催化环异构区域选择性的确定邻- (苯乙炔基)苯乙烯。根据我们的计算,无论使用α-取代的或β,β-二取代的邻-(苯基乙炔基)苯乙烯,一种新颖且不寻常的消除质子的反应途径均涉及四中心三电子中间体,而不是生成观察到HSbF 6。由于电子效应,对于α-取代的邻-(苯基乙炔基)苯乙烯,环的扩展途径比消除途径更有利。通过使用β,β-二取代的邻-(苯基乙炔基)苯乙烯,一种涉及四中心三电子中间体的消除途径,提供了茚基衍生物。根据我们的计算结果和实验事实,标题反应的区域选择性主要受电子效应而非空间效应控制。理论结果不仅很好地使实验观察合理化,而且还提供了对反应细节的见解。
更新日期:2018-04-02
中文翻译:

黄金(我)的催化的环异构邻- (炔基)苯乙烯:的SBF的至关重要的作用DFT分析6 -在消除质子†
这项研究报告通过的SbF发挥的重要作用的第一个理论依据6 -在黄金催化环异构区域选择性的确定邻- (苯乙炔基)苯乙烯。根据我们的计算,无论使用α-取代的或β,β-二取代的邻-(苯基乙炔基)苯乙烯,一种新颖且不寻常的消除质子的反应途径均涉及四中心三电子中间体,而不是生成观察到HSbF 6。由于电子效应,对于α-取代的邻-(苯基乙炔基)苯乙烯,环的扩展途径比消除途径更有利。通过使用β,β-二取代的邻-(苯基乙炔基)苯乙烯,一种涉及四中心三电子中间体的消除途径,提供了茚基衍生物。根据我们的计算结果和实验事实,标题反应的区域选择性主要受电子效应而非空间效应控制。理论结果不仅很好地使实验观察合理化,而且还提供了对反应细节的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号