当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient C–C coupling of bio-based furanics and carbonyl compounds to liquid hydrocarbon precursors over lignosulfonate derived acidic carbocatalysts†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7cy02601c Lakhya Jyoti Konwar 1, 2, 3, 4, 5 , Ajaikumar Samikannu 1, 2, 3, 4, 5 , Päivi Mäki-Arvela 6, 7, 8, 9, 10 , Jyri-Pekka Mikkola 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1039/c7cy02601c Lakhya Jyoti Konwar 1, 2, 3, 4, 5 , Ajaikumar Samikannu 1, 2, 3, 4, 5 , Päivi Mäki-Arvela 6, 7, 8, 9, 10 , Jyri-Pekka Mikkola 1, 2, 3, 4, 5
Affiliation
|
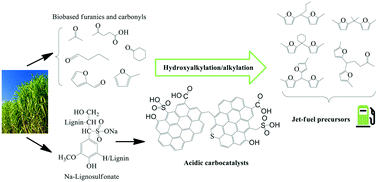
This paper demonstrates the catalytic potential of novel Na-lignosulfonate (LS) derived meso/macroporous solid protonic acids upon C–C coupling of bio-based furanics and carbonyl compounds. The materials demonstrated catalytic activity for solventless hydroxyalkylation/alkylation (HAA) of 2-methylfuran with furfural, acetone, butanal, cyclohexanone, levulinic acid and α-angelica lactone under mild reaction conditions (50–60 °C) producing branched-chain C12–C16 hydrocarbon precursors in yields approaching 96%. Moreover, the carbon materials exhibiting high total acidity (6–6.4 mmol g−1) outperformed sulfonic acid resins (Amberlyst®70, Amberlite®IR120 and LS resin), zeolites and liquid acids (p-toluenesulfonic acid, acetic acid and phenol). In fact, the most active carbocatalyst (60LS40PS350H+) exhibited the same turnover frequency as p-toluenesulfonic acid (186 h−1) upon furfural conversion but with an improved HAA product yield (up to 88%) and reusability, maintaining 98% of its original activity up to seven reaction cycles. The observed catalytic activity and operational stability of the LS derived acidic carbocatalysts were attributed to the strongly Brønsted acidic –SO3H groups covalently incorporated into their structural carbon framework and the promotional effects of hydrophilic surface functional groups (–COOH and –OH) favoring adsorption of oxygenated reactant molecules.
中文翻译:

通过木质素磺酸盐衍生的酸性碳催化剂将生物基呋喃化合物和羰基化合物与液态烃前体进行高效的C–C耦合†
本文证明了生物基呋喃化合物和羰基化合物的C–C偶联后,新的由木质素磺酸钠(LS)衍生的中/大分子固体质子酸的催化潜力。该材料在温和的反应条件下(50–60°C)对糠醛,丙酮,丁醛,环己酮,乙酰丙酸和α-当归内酯的2-甲基呋喃无溶剂羟烷基化/烷基化(HAA)表现出催化活性,产生支链C12– C16烃前体的收率接近96%。此外,表现出高总酸度(6-6.4 mmol g -1)的碳材料的性能优于磺酸树脂(Amberlyst®70,Amberlite®IR120和LS树脂),沸石和液态酸(p-甲苯磺酸,乙酸和苯酚)。实际上,糠醛转化后,最具活性的碳催化剂(60LS40PS350H +)表现出与对甲苯磺酸(186 h -1)相同的周转频率,但具有更高的HAA产物收率(高达88%)和可重复使用性,可保持98%的碳氢化合物利用率。其原始活性最多可达到七个反应周期。观察到的LS衍生的酸性碳催化剂的催化活性和操作稳定性归因于共价结合到结构碳骨架中的强烈Brønsted酸性–SO 3 H基团以及亲水性表面官能团(–COOH和–OH)的促进作用,有利于吸附含氧的反应物分子。
更新日期:2018-04-02
中文翻译:

通过木质素磺酸盐衍生的酸性碳催化剂将生物基呋喃化合物和羰基化合物与液态烃前体进行高效的C–C耦合†
本文证明了生物基呋喃化合物和羰基化合物的C–C偶联后,新的由木质素磺酸钠(LS)衍生的中/大分子固体质子酸的催化潜力。该材料在温和的反应条件下(50–60°C)对糠醛,丙酮,丁醛,环己酮,乙酰丙酸和α-当归内酯的2-甲基呋喃无溶剂羟烷基化/烷基化(HAA)表现出催化活性,产生支链C12– C16烃前体的收率接近96%。此外,表现出高总酸度(6-6.4 mmol g -1)的碳材料的性能优于磺酸树脂(Amberlyst®70,Amberlite®IR120和LS树脂),沸石和液态酸(p-甲苯磺酸,乙酸和苯酚)。实际上,糠醛转化后,最具活性的碳催化剂(60LS40PS350H +)表现出与对甲苯磺酸(186 h -1)相同的周转频率,但具有更高的HAA产物收率(高达88%)和可重复使用性,可保持98%的碳氢化合物利用率。其原始活性最多可达到七个反应周期。观察到的LS衍生的酸性碳催化剂的催化活性和操作稳定性归因于共价结合到结构碳骨架中的强烈Brønsted酸性–SO 3 H基团以及亲水性表面官能团(–COOH和–OH)的促进作用,有利于吸附含氧的反应物分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号