当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pulsed Hydrogen–Deuterium Exchange Illuminates the Aggregation Kinetics of α-Synuclein, the Causative Agent for Parkinson’s Disease
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acschemneuro.8b00052 Eva Illes-Toth 1 , Don L. Rempel 1 , Michael L. Gross 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acschemneuro.8b00052 Eva Illes-Toth 1 , Don L. Rempel 1 , Michael L. Gross 1
Affiliation
|
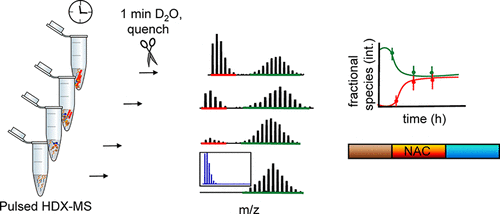
α-Synuclein (aS) forms toxic intermediates ranging from small oligomers and protofibrils to large amyloid fibrils. Understanding the time course of aS fibril formation and the role played by its regions is critical for therapeutic intervention. Here, we used pulsed hydrogen–deuterium exchange and mass spectrometry (HDX-MS) for the first time to probe kinetic intermediates of the full aS aggregation in vitro, achieving kinetic snapshots containing spatially resolved protein information about critical stages. Monitoring the resultant mass shifts shows distinct binomial abundances for two main exchange profiles: one that represents a fast-exchanging, solvent-accessible species and another with a more protected nature. We show using a series of proteolytic peptides from the full protein that self-association is most pronounced in the non-amyloid-β-component region and less so for either terminus. The N-terminus, however, shows a minor protected population at mid- and late times, whereas the C-terminus shows predominantly unimodal HDX, indicating that these regions are devoid of any large conformational rearrangements. Focusing on the hydrophobic core, we confirmed and modeled the different isotopic distributions and calculated their relative fractions to discern their individual contributions. The data fitting reports respective t1/2 values, which are nearly identical and do not depend on location. We followed the aggregation by complementary transmission electron microscopy to observe the morphology of aggregates and circular dichroism to assess changes in secondary structure. Our results provide a detailed picture of aS aggregation in vitro and demonstrate that HDX-MS offers unique spatially resolved, coexisting kinetic intermediates in solution. This new platform is suitable for testing promising inhibitors of aS aggregation.
中文翻译:

脉冲氢-氘交换阐明了帕金森氏病的病原体α-突触核蛋白的聚集动力学
α-突触核蛋白(aS)形成有毒的中间体,从小的寡聚物和原纤维到大的淀粉样原纤维。了解aS原纤维形成的时间过程及其区域所起的作用对于治疗干预至关重要。在这里,我们首次使用脉冲氢-氘交换和质谱(HDX-MS)来体外探究完整aS聚集的动力学中间体,从而获得动力学快照,其中包含有关关键阶段的空间分辨蛋白质信息。监测由此产生的质量变化,可发现两种主要交换模式的二项式丰度:一种代表快速交换,溶剂可及的物种,另一种具有更受保护的性质。我们显示了使用来自完整蛋白的一系列蛋白水解肽,其自缔合在非淀粉样β-组分区域中最为明显,而对于任一末端而言则更少。但是,N端在中后期显示了少量受保护的种群,而C端则主要显示了单峰HDX,表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自 表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自 表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自t 1/2值,它们几乎是相同的,并且与位置无关。我们通过互补透射电子显微镜观察聚集体,观察聚集体的形态和圆二色性,以评估二级结构的变化。我们的结果提供了体外aS聚集的详细图片,并证明HDX-MS在溶液中提供了独特的空间分辨的,共存的动力学中间体。这个新平台适用于测试有前途的aS聚集抑制剂。
更新日期:2018-03-30
中文翻译:

脉冲氢-氘交换阐明了帕金森氏病的病原体α-突触核蛋白的聚集动力学
α-突触核蛋白(aS)形成有毒的中间体,从小的寡聚物和原纤维到大的淀粉样原纤维。了解aS原纤维形成的时间过程及其区域所起的作用对于治疗干预至关重要。在这里,我们首次使用脉冲氢-氘交换和质谱(HDX-MS)来体外探究完整aS聚集的动力学中间体,从而获得动力学快照,其中包含有关关键阶段的空间分辨蛋白质信息。监测由此产生的质量变化,可发现两种主要交换模式的二项式丰度:一种代表快速交换,溶剂可及的物种,另一种具有更受保护的性质。我们显示了使用来自完整蛋白的一系列蛋白水解肽,其自缔合在非淀粉样β-组分区域中最为明显,而对于任一末端而言则更少。但是,N端在中后期显示了少量受保护的种群,而C端则主要显示了单峰HDX,表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自 表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自 表明这些区域没有任何大的构象重排。着眼于疏水核,我们确认并建模了不同的同位素分布,并计算了它们的相对分数,以辨别它们的个体贡献。数据拟合报告各自t 1/2值,它们几乎是相同的,并且与位置无关。我们通过互补透射电子显微镜观察聚集体,观察聚集体的形态和圆二色性,以评估二级结构的变化。我们的结果提供了体外aS聚集的详细图片,并证明HDX-MS在溶液中提供了独特的空间分辨的,共存的动力学中间体。这个新平台适用于测试有前途的aS聚集抑制剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号