当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paradoxical Effect of Trehalose on the Aggregation of α-Synuclein: Expedites Onset of Aggregation yet Reduces Fibril Load
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acschemneuro.8b00056 Nidhi Katyal 1 , Manish Agarwal 1 , Raktim Sen 1 , Vinay Kumar 1 , Shashank Deep 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acschemneuro.8b00056 Nidhi Katyal 1 , Manish Agarwal 1 , Raktim Sen 1 , Vinay Kumar 1 , Shashank Deep 1
Affiliation
|
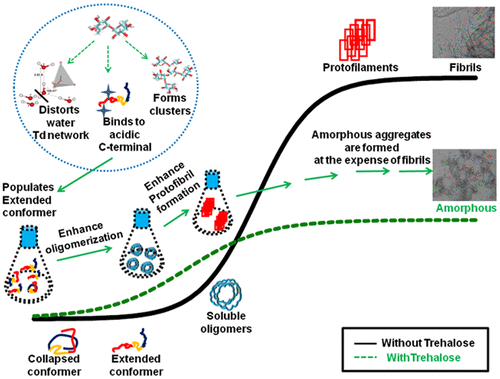
Aggregation of α-synuclein is closely connected to the pathology of Parkinson’s disease. The phenomenon involves multiple steps, commenced by partial misfolding and eventually leading to mature amyloid fibril formation. Trehalose, a widely accepted osmolyte, has been shown previously to inhibit aggregation of various globular proteins owing to its ability to prevent the initial unfolding of protein. In this study, we have examined if it behaves in a similar fashion with intrinsically disordered protein α-synuclein and possesses the potential to act as therapeutic agent against Parkinson’s disease. It was observed experimentally that samples coincubated with trehalose fibrillate faster compared to the case in its absence. Molecular dynamics simulations suggested that this initial acceleration is manifestation of trehalose’s tendency to perturb the conformational transitions between different conformers of monomeric protein. It stabilizes the aggregation prone “extended” conformer of α-synuclein, by binding to its exposed acidic residues of the C terminus. It also favors the β-rich oligomers once formed. Interestingly, the total fibrils formed are still promisingly less since it accelerates the competing pathway toward formation of amorphous aggregates.
中文翻译:

海藻糖对α-突触核蛋白聚集的悖论作用:加快聚集的发作,但减少原纤维的负荷。
α-突触核蛋白的聚集与帕金森氏病的病理密切相关。该现象涉及多个步骤,从部分错误折叠开始,最终导致成熟的淀粉样蛋白原纤维形成。海藻糖,一种被广泛接受的渗透压剂,由于其具有阻止蛋白质最初展开的能力,因此先前已显示出抑制各种球状蛋白质聚集的作用。在这项研究中,我们检查了它与内在失调的蛋白质α-突触核蛋白是否具有相似的行为,并具有充当针对帕金森氏病的治疗剂的潜力。实验上观察到,与不存在海藻糖原纤维共孵育的样品相比,与海藻糖原纤维共孵育的样品更快。分子动力学模拟表明,这种初始加速是海藻糖倾向于干扰单体蛋白不同构象体之间构象转变的趋势的体现。它通过与C末端暴露的酸性残基结合,稳定了α-突触核蛋白的易于聚集的“延伸”构象体。一旦形成,它也有利于富含β的低聚物。有趣的是,形成的总原纤维仍有望减少,因为它加速了形成无定形聚集体的竞争途径。
更新日期:2018-03-30
中文翻译:

海藻糖对α-突触核蛋白聚集的悖论作用:加快聚集的发作,但减少原纤维的负荷。
α-突触核蛋白的聚集与帕金森氏病的病理密切相关。该现象涉及多个步骤,从部分错误折叠开始,最终导致成熟的淀粉样蛋白原纤维形成。海藻糖,一种被广泛接受的渗透压剂,由于其具有阻止蛋白质最初展开的能力,因此先前已显示出抑制各种球状蛋白质聚集的作用。在这项研究中,我们检查了它与内在失调的蛋白质α-突触核蛋白是否具有相似的行为,并具有充当针对帕金森氏病的治疗剂的潜力。实验上观察到,与不存在海藻糖原纤维共孵育的样品相比,与海藻糖原纤维共孵育的样品更快。分子动力学模拟表明,这种初始加速是海藻糖倾向于干扰单体蛋白不同构象体之间构象转变的趋势的体现。它通过与C末端暴露的酸性残基结合,稳定了α-突触核蛋白的易于聚集的“延伸”构象体。一旦形成,它也有利于富含β的低聚物。有趣的是,形成的总原纤维仍有望减少,因为它加速了形成无定形聚集体的竞争途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号