当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Transformation of Two-Line Ferrihydrite into Crystalline Products: Effect of pH and Media (Sulfate versus Nitrate)
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00001 Danni Zhang 1 , Shaofeng Wang 1 , Ying Wang 1, 2 , Mario A. Gomez 3 , Yihang Duan 1, 2 , Yongfeng Jia 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00001 Danni Zhang 1 , Shaofeng Wang 1 , Ying Wang 1, 2 , Mario A. Gomez 3 , Yihang Duan 1, 2 , Yongfeng Jia 1
Affiliation
|
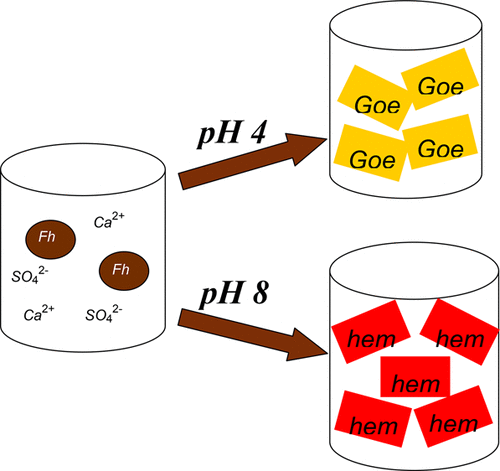
Two-line ferrihydrite (Fh), ubiquitous in soils, groundwater, and aquatic sediments, may serve as an important sink for sequestering trace metals, metalloids, and organic matter via adsorption/coprecipitation due to its high surface area and reactivity. Although considerable attention has been paid to the transformation process of this thermodynamically metastable solid, little is known about the transformation products, the crystallization rates, or the transformation routes of two-line Fh in sulfate- and calcium-rich environments. This work systematically investigates the transformation of 2-line ferrihydrite produced by using different neutralization reagents (CaO vs NaOH) at different pHs (4 and 8), temperatures (25 °C, 40 °C, and 80 °C), and media (sulfate vs nitrate). X-ray diffraction, Raman, Fourier transform infrared spectroscopy, and chemical extraction were employed to characterize the transformed solids as well as the crystallization rate of 2-line Fh. The results show that the crystallization products in nitrate media include hematite (major) and goethite (minor) under acidic conditions (pH 4) and only hematite under alkaline conditions (pH 8) and are independent of the neutralization reagent used. By contrast, in sulfate media, goethite is the dominant product under slightly acidic conditions (pH 4) with 6-line ferrihydrite as the intermediate product, whereas under slightly alkaline conditions the formation of hematite is favored. Chemical extraction results indicate that the transformation process of those CaO-neutralized solids precipitated at pH 8 is intensely retarded by calcium ion. The results suggest that neutralization reagents (calcium ion), as well as the reaction media (sulfate ion), play an important role in the 2-line ferrihydrite crystallization process.
中文翻译:

两线铁水合物转变为结晶产物:pH和介质(硫酸盐与硝酸盐)的影响
在土壤,地下水和水生沉积物中普遍存在的两线铁水铁矿(Fh),由于其高表面积和反应性,可以通过吸附/共沉淀作用来隔离痕量金属,准金属和有机物,是一个重要的接收器。尽管已经对该热力学亚稳态固体的转化过程给予了极大的关注,但对于富含硫酸盐和钙的环境中的转化产物,结晶速率或两线式Fh的转化途径知之甚少。这项工作系统地研究了在不同pH(4和8),温度(25°C,40°C和80°C)和介质(硫酸盐对硝酸盐)。X射线衍射,拉曼光谱,傅立叶变换红外光谱,通过化学萃取和化学萃取来表征转化的固体以及2线Fh的结晶速率。结果表明,硝酸盐介质中的结晶产物包括酸性条件(pH 4)下的赤铁矿(主要)和针铁矿(次要),以及碱性条件下(pH 8)的仅赤铁矿,并且与所使用的中和试剂无关。相比之下,在硫酸盐介质中,针铁矿是在弱酸性条件下(pH 4)的主要产物,其中六线亚铁酸盐作为中间产物,而在弱碱性条件下则有利于赤铁矿的形成。化学提取结果表明,钙离子强烈阻碍了pH 8沉淀的那些CaO中和的固体的转化过程。结果表明,中和试剂(钙离子),
更新日期:2018-03-29
中文翻译:

两线铁水合物转变为结晶产物:pH和介质(硫酸盐与硝酸盐)的影响
在土壤,地下水和水生沉积物中普遍存在的两线铁水铁矿(Fh),由于其高表面积和反应性,可以通过吸附/共沉淀作用来隔离痕量金属,准金属和有机物,是一个重要的接收器。尽管已经对该热力学亚稳态固体的转化过程给予了极大的关注,但对于富含硫酸盐和钙的环境中的转化产物,结晶速率或两线式Fh的转化途径知之甚少。这项工作系统地研究了在不同pH(4和8),温度(25°C,40°C和80°C)和介质(硫酸盐对硝酸盐)。X射线衍射,拉曼光谱,傅立叶变换红外光谱,通过化学萃取和化学萃取来表征转化的固体以及2线Fh的结晶速率。结果表明,硝酸盐介质中的结晶产物包括酸性条件(pH 4)下的赤铁矿(主要)和针铁矿(次要),以及碱性条件下(pH 8)的仅赤铁矿,并且与所使用的中和试剂无关。相比之下,在硫酸盐介质中,针铁矿是在弱酸性条件下(pH 4)的主要产物,其中六线亚铁酸盐作为中间产物,而在弱碱性条件下则有利于赤铁矿的形成。化学提取结果表明,钙离子强烈阻碍了pH 8沉淀的那些CaO中和的固体的转化过程。结果表明,中和试剂(钙离子),











































 京公网安备 11010802027423号
京公网安备 11010802027423号