Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evolutionary Convergence of Pathway-Specific Enzyme Expression Stoichiometry.
Cell ( IF 45.5 ) Pub Date : 2018-Apr-19 , DOI: 10.1016/j.cell.2018.03.007 Jean-Benoît Lalanne 1 , James C Taggart 2 , Monica S Guo 2 , Lydia Herzel 2 , Ariel Schieler 2 , Gene-Wei Li 2
Cell ( IF 45.5 ) Pub Date : 2018-Apr-19 , DOI: 10.1016/j.cell.2018.03.007 Jean-Benoît Lalanne 1 , James C Taggart 2 , Monica S Guo 2 , Lydia Herzel 2 , Ariel Schieler 2 , Gene-Wei Li 2
Affiliation
|
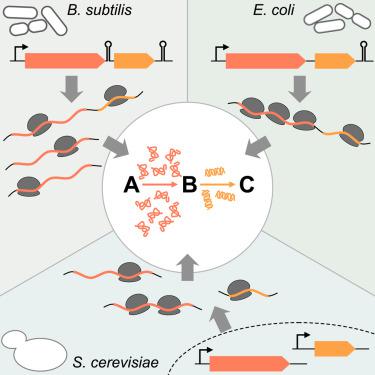
Coexpression of proteins in response to pathway-inducing signals is the founding paradigm of gene regulation. However, it remains unexplored whether the relative abundance of co-regulated proteins requires precise tuning. Here, we present large-scale analyses of protein stoichiometry and corresponding regulatory strategies for 21 pathways and 67-224 operons in divergent bacteria separated by 0.6-2 billion years. Using end-enriched RNA-sequencing (Rend-seq) with single-nucleotide resolution, we found that many bacterial gene clusters encoding conserved pathways have undergone massive divergence in transcript abundance and architectures via remodeling of internal promoters and terminators. Remarkably, these evolutionary changes are compensated post-transcriptionally to maintain preferred stoichiometry of protein synthesis rates. Even more strikingly, in eukaryotic budding yeast, functionally analogous proteins that arose independently from bacterial counterparts also evolved to convergent in-pathway expression. The broad requirement for exact protein stoichiometries despite regulatory divergence provides an unexpected principle for building biological pathways both in nature and for synthetic activities.
中文翻译:

途径特异性酶表达化学计量的进化收敛。
响应通路诱导信号的蛋白质共表达是基因调控的基础范例。然而,共调节蛋白的相对丰度是否需要精确调节仍有待探索。在这里,我们对相距 6-2 亿年的不同细菌中 21 条途径和 67-224 个操纵子的蛋白质化学计量和相应的调控策略进行了大规模分析。使用单核苷酸分辨率的末端富集 RNA 测序 (Rend-seq),我们发现许多编码保守通路的细菌基因簇通过内部启动子和终止子的重塑,在转录本丰度和结构上发生了巨大差异。值得注意的是,这些进化变化在转录后得到补偿,以维持蛋白质合成速率的首选化学计量。更引人注目的是,在真核芽殖酵母中,独立于细菌对应物产生的功能相似的蛋白质也进化为会聚的途径内表达。尽管存在监管分歧,但对精确蛋白质化学计量的广泛要求为构建自然界和合成活动的生物途径提供了意想不到的原则。
更新日期:2018-04-26
中文翻译:

途径特异性酶表达化学计量的进化收敛。
响应通路诱导信号的蛋白质共表达是基因调控的基础范例。然而,共调节蛋白的相对丰度是否需要精确调节仍有待探索。在这里,我们对相距 6-2 亿年的不同细菌中 21 条途径和 67-224 个操纵子的蛋白质化学计量和相应的调控策略进行了大规模分析。使用单核苷酸分辨率的末端富集 RNA 测序 (Rend-seq),我们发现许多编码保守通路的细菌基因簇通过内部启动子和终止子的重塑,在转录本丰度和结构上发生了巨大差异。值得注意的是,这些进化变化在转录后得到补偿,以维持蛋白质合成速率的首选化学计量。更引人注目的是,在真核芽殖酵母中,独立于细菌对应物产生的功能相似的蛋白质也进化为会聚的途径内表达。尽管存在监管分歧,但对精确蛋白质化学计量的广泛要求为构建自然界和合成活动的生物途径提供了意想不到的原则。











































 京公网安备 11010802027423号
京公网安备 11010802027423号