当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed C–O Coupling of a Sterically Hindered Secondary Alcohol with an Aryl Bromide and Significant Purity Upgrade in the API Step
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acs.oprd.8b00022 Ian S. Young 1 , Eric M. Simmons 1 , Michaël D. B. Fenster 1 , Jason J. Zhu 1 , Kishta R. Katipally 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acs.oprd.8b00022 Ian S. Young 1 , Eric M. Simmons 1 , Michaël D. B. Fenster 1 , Jason J. Zhu 1 , Kishta R. Katipally 1
Affiliation
|
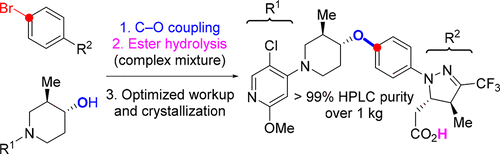
The final two steps used to prepare greater than 1 kg of a compound evaluated as a treatment for type 2 diabetes are reported. The application of a palladium-catalyzed C–O coupling presented significant challenges due to the nature of the reactants, impurities produced, and noncrystalline coupling intermediate. Process development was able to address these limitations and enable production of kilogram quantities of the active pharmaceutical ingredient (API) in greater efficiency than a Mitsunobu reaction for formation of the key bond. The development of a sequence that telescopes the coupling with the subsequent ester hydrolysis to yield the API and the workup and final product crystallization necessary to produce high-quality drug substance without the need of column chromatography are discussed.
中文翻译:

钯催化的芳族溴化物与位阻受阻二级醇的C-O偶联,并且在API步骤中显着提高了纯度
据报道,用于制备大于1千克化合物的最后两个步骤被评估为2型糖尿病的治疗药物。由于反应物的性质,产生的杂质和非晶态的偶联中间体,钯催化的C-O偶联的应用提出了严峻的挑战。工艺开发能够解决这些局限性,并能够以比Mitsunobu反应更高的效率生产千克量的活性药物成分(API),以形成关键键。讨论了通过伸缩与随后的酯水解以产生API的偶联序列的开发,以及无需柱色谱法即可生产高质量药物所需的后处理和最终产物结晶的情况。
更新日期:2018-03-29
中文翻译:

钯催化的芳族溴化物与位阻受阻二级醇的C-O偶联,并且在API步骤中显着提高了纯度
据报道,用于制备大于1千克化合物的最后两个步骤被评估为2型糖尿病的治疗药物。由于反应物的性质,产生的杂质和非晶态的偶联中间体,钯催化的C-O偶联的应用提出了严峻的挑战。工艺开发能够解决这些局限性,并能够以比Mitsunobu反应更高的效率生产千克量的活性药物成分(API),以形成关键键。讨论了通过伸缩与随后的酯水解以产生API的偶联序列的开发,以及无需柱色谱法即可生产高质量药物所需的后处理和最终产物结晶的情况。









































 京公网安备 11010802027423号
京公网安备 11010802027423号