当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sunlight-charged heterojunction TiO2 and WO3 particle-embedded inorganic membranes for night-time environmental applications
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1039/c7pp00451f Dong Suk Han 1, 2, 3, 4 , Rand Elshorafa 5, 6, 7, 8, 9 , Sun Hee Yoon 1, 2, 3, 4 , Seonghun Kim 10, 11, 12, 13 , Hyunwoong Park 10, 11, 12, 13 , Ahmed Abdel-Wahab 1, 2, 3, 4
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1039/c7pp00451f Dong Suk Han 1, 2, 3, 4 , Rand Elshorafa 5, 6, 7, 8, 9 , Sun Hee Yoon 1, 2, 3, 4 , Seonghun Kim 10, 11, 12, 13 , Hyunwoong Park 10, 11, 12, 13 , Ahmed Abdel-Wahab 1, 2, 3, 4
Affiliation
|
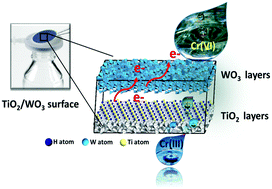
A metal oxide-heterojunction photocatalyst is developed to harvest sunlight, store the energy in electrons, and apply the stored energy in water treatment. Light-absorbing nanoparticular and nanotubular TiO2 are hybridized with electron-storing WO3 at different weight ratios of TiO2 to WO3 (e.g., TW25 represents a composite of 25 wt% TiO2 and 75 wt% WO3). The ability of the TW composite to utilize the stored electrons is examined for the reduction of hexavalent chromium (Cr(VI)). In the photoelectrochemical (PEC) tests, irradiation using simulated sunlight (AM 1.5, 100 mW cm−2) leads to a rapid shift in the open-circuit potential (OCP) of the TW electrodes to the negative potential region (photocharging process). The termination of irradiation causes a gradual shift of the OCP to the positive potential region over 20 h (discharging process). Spiked Cr(VI) added to the solution with pre-photocharged TW electrodes is efficiently removed; the kinetics of this process depends on the TW composition (25, 50, or 75 wt%), TiO2 morphology (particular or tubular), initial Cr(VI) concentration (0.125 or 0.25 ppm), and whether the conditions are aerated or non-aerated. Based on this knowledge, TW composite-embedded inorganic membranes are synthesized and charged using sunlight. For Cr(VI) removal, single-pass and continuous circulation filtration systems are employed. The fraction of Cr(VI) removed from the circulation system is ∼30% in 4 h, which is 1.5 times that removed using the single-pass filtration system (∼20%). An X-ray photoelectron spectroscopy analysis of the TW membranes used for Cr(VI) removal reveals that Cr is not sorbed in the membrane. The W(VI) in WO3 is partially reduced to W(6−x)+ upon photocharging and is oxidized during the reduction of Cr(VI), leading to the co-existence of W6+ and W(6−x)+.
中文翻译:

用于夜间环境应用 的带日光的异质结TiO 2和WO 3颗粒嵌入无机膜
开发了一种金属氧化物-异质结光催化剂,以收集阳光,将能量存储在电子中,并将存储的能量应用于水处理。光吸收纳米颗粒和纳米管的TiO 2杂交与电子存储WO 3在二氧化钛的不同重量比2至WO 3(例如,TW25表示复合材料的25重量%的TiO 2和75重量%的WO 3)。检查了TW复合材料利用存储的电子的能力,以还原六价铬(Cr(VI))。在光电化学(PEC)测试中,使用模拟阳光(AM 1.5,100 mW cm -2)导致TW电极的开路电势(OCP)迅速移向负电势区域(光充电过程)。辐照终止会导致OCP在20小时内逐渐移至正电位区域(放电过程)。通过预光充电的TW电极可以有效地去除添加到溶液中的尖峰Cr(VI)。此过程的动力学取决于TW组成(25、50或75 wt%),TiO 2形态(特殊或管状),初始Cr(VI)浓度(0.125或0.25 ppm)以及条件是否为充气或充气。未充气。基于该知识,使用阳光将TW复合材料包埋的无机膜合成并带电。铬(VI)去除,采用单程和连续循环过滤系统。从循环系统中去除的Cr(VI)分数在4小时内约为30%,是使用单程过滤系统去除的Cr(VI)的1.5倍(约为20%)。对用于去除Cr(VI)的TW膜的X射线光电子能谱分析表明,Cr没有吸附在膜中。WO 3中的W(VI)在光充电时部分还原为W (6- x)+,并在Cr(VI)还原过程中被氧化,导致W 6+和W (6- x)共存。+。
更新日期:2018-03-29
中文翻译:

用于夜间环境应用 的带日光的异质结TiO 2和WO 3颗粒嵌入无机膜
开发了一种金属氧化物-异质结光催化剂,以收集阳光,将能量存储在电子中,并将存储的能量应用于水处理。光吸收纳米颗粒和纳米管的TiO 2杂交与电子存储WO 3在二氧化钛的不同重量比2至WO 3(例如,TW25表示复合材料的25重量%的TiO 2和75重量%的WO 3)。检查了TW复合材料利用存储的电子的能力,以还原六价铬(Cr(VI))。在光电化学(PEC)测试中,使用模拟阳光(AM 1.5,100 mW cm -2)导致TW电极的开路电势(OCP)迅速移向负电势区域(光充电过程)。辐照终止会导致OCP在20小时内逐渐移至正电位区域(放电过程)。通过预光充电的TW电极可以有效地去除添加到溶液中的尖峰Cr(VI)。此过程的动力学取决于TW组成(25、50或75 wt%),TiO 2形态(特殊或管状),初始Cr(VI)浓度(0.125或0.25 ppm)以及条件是否为充气或充气。未充气。基于该知识,使用阳光将TW复合材料包埋的无机膜合成并带电。铬(VI)去除,采用单程和连续循环过滤系统。从循环系统中去除的Cr(VI)分数在4小时内约为30%,是使用单程过滤系统去除的Cr(VI)的1.5倍(约为20%)。对用于去除Cr(VI)的TW膜的X射线光电子能谱分析表明,Cr没有吸附在膜中。WO 3中的W(VI)在光充电时部分还原为W (6- x)+,并在Cr(VI)还原过程中被氧化,导致W 6+和W (6- x)共存。+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号