Science of the Total Environment ( IF 8.2 ) Pub Date : 2018-03-28 , DOI: 10.1016/j.scitotenv.2018.03.181 Mingizem Gashaw Seid , Kangwoo Cho , Changha Lee , Hyun-Mee Park , Seok Won Hong
|
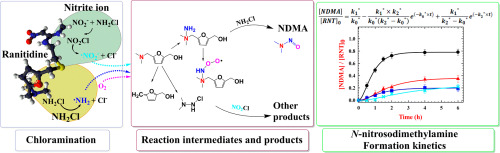
Ranitidine (RNT) has been an important tertiary amine precursor of N-nitrosodimethylamine (NDMA) in chlorine-based water treatment, due to reaction with monochloramine (NH2Cl) with exceptionally high molar yields up to 90%. This study examined the effects of nitrite ions (NO2−) on the kinetics of NDMA formation during the chloramination of RNT under variable concentrations of dissolved oxygen (DO, 0.7–7.5 mg/L), RNT (5–30 μM), NH2Cl (5–20 mM), NO2− or NO3− (0–2 mM) and pH (5.6–8.6). In the absence of the NO2−, the ultimate molar yield of NDMA after 6 h of reaction was primarily influenced by [DO] and pH, while marginally affected by initial [RNT] and [NH2Cl]. A kinetic model, prepared in accordance with the reaction sequence of NDMA formation, suggested that the rate determining step was accelerated with increasing [NH2Cl]0, [DO], and pH. A Kinetic study together with ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometer (UPLC-Q-TOF MS) and gas chromatography (GC)/TOF MS analyses in parallel demonstrated that the nitrite ion inhibited the nucleophilic substitution of the terminal amine on NH2Cl, and reduced the pseudo-steady state concentration of N-peroxyl radicals, significantly decreasing the ultimate yields of NDMA.
中文翻译:

亚硝酸根离子可减轻雷尼替丁氯化过程中N-亚硝基二甲胺(NDMA)的形成
雷尼替丁(RNT)在氯基水处理中一直是N-亚硝基二甲胺(NDMA)的重要叔胺前体,这是因为它与一氯胺(NH 2 Cl)反应的摩尔产率高达90%,异常高。本研究亚硝酸根离子的效果(NO 2 - RNT的溶解氧的浓度可变(DO,0.7-7.5毫克/升),RNT(5-30μM),NH下氯胺期间的NDMA形成的动力学)2氯(5-20毫米),NO 2 -或NO 3 -(0-2毫米)和pH值(5.6-8.6)。在没有NO 2的情况下-,反应6小时后NDMA的最终摩尔产率主要受[DO]和pH的影响,而受起始[RNT]和[NH 2 Cl]的影响很小。根据NDMA形成的反应顺序制备的动力学模型表明,速率确定步骤随着[NH 2 Cl] 0,[DO]和pH的增加而加速。动力学研究与超高效液相色谱-四极杆飞行时间质谱仪(UPLC-Q-TOF MS)和气相色谱仪(GC)/ TOF MS并行分析表明,亚硝酸根离子抑制了末端的亲核取代胺在NH 2 Cl上,降低了N的拟稳态浓度-过氧自由基,大大降低了NDMA的最终收率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号