Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Architecture of the human GATOR1 and GATOR1–Rag GTPases complexes
Nature ( IF 50.5 ) Pub Date : 2018-03-28 , DOI: 10.1038/nature26158 Kuang Shen 1, 2, 3, 4 , Rick K Huang 5 , Edward J Brignole 2, 6 , Kendall J Condon 1, 2, 3, 4 , Max L Valenstein 1, 2, 3, 4 , Lynne Chantranupong 1, 2, 3, 4 , Aimaiti Bomaliyamu 1 , Abigail Choe 1 , Chuan Hong 5 , Zhiheng Yu 5 , David M Sabatini 1, 2, 3, 4
Nature ( IF 50.5 ) Pub Date : 2018-03-28 , DOI: 10.1038/nature26158 Kuang Shen 1, 2, 3, 4 , Rick K Huang 5 , Edward J Brignole 2, 6 , Kendall J Condon 1, 2, 3, 4 , Max L Valenstein 1, 2, 3, 4 , Lynne Chantranupong 1, 2, 3, 4 , Aimaiti Bomaliyamu 1 , Abigail Choe 1 , Chuan Hong 5 , Zhiheng Yu 5 , David M Sabatini 1, 2, 3, 4
Affiliation
|
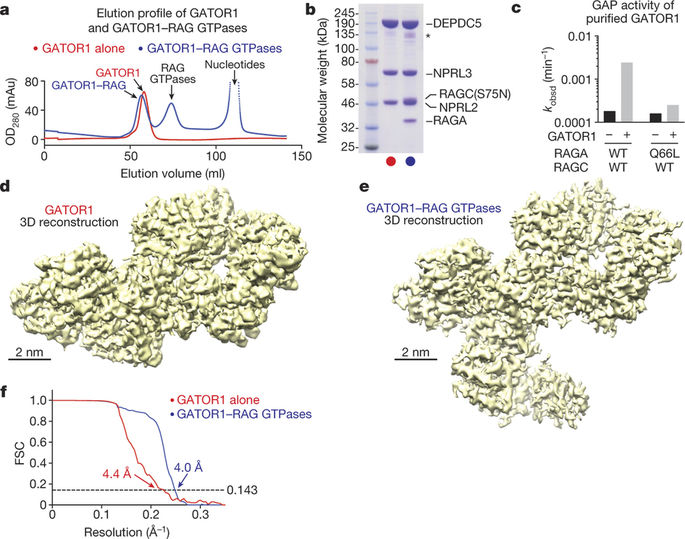
Nutrients, such as amino acids and glucose, signal through the Rag GTPases to activate mTORC1. The GATOR1 protein complex—comprising DEPDC5, NPRL2 and NPRL3—regulates the Rag GTPases as a GTPase-activating protein (GAP) for RAGA; loss of GATOR1 desensitizes mTORC1 signalling to nutrient starvation. GATOR1 components have no sequence homology to other proteins, so the function of GATOR1 at the molecular level is currently unknown. Here we used cryo-electron microscopy to solve structures of GATOR1 and GATOR1–Rag GTPases complexes. GATOR1 adopts an extended architecture with a cavity in the middle; NPRL2 links DEPDC5 and NPRL3, and DEPDC5 contacts the Rag GTPase heterodimer. Biochemical analyses reveal that our GATOR1–Rag GTPases structure is inhibitory, and that at least two binding modes must exist between the Rag GTPases and GATOR1. Direct interaction of DEPDC5 with RAGA inhibits GATOR1-mediated stimulation of GTP hydrolysis by RAGA, whereas weaker interactions between the NPRL2–NPRL3 heterodimer and RAGA execute GAP activity. These data reveal the structure of a component of the nutrient-sensing mTORC1 pathway and a non-canonical interaction between a GAP and its substrate GTPase.
中文翻译:

人类 GATOR1 和 GATOR1-Rag GTPases 复合物的结构
氨基酸和葡萄糖等营养物质通过 Rag GTPases 发出信号来激活 mTORC1。 GATOR1 蛋白复合物(包含 DEPDC5、NPRL2 和 NPRL3)作为 RAGA 的 GTP 酶激活蛋白 (GAP) 调节 Rag GTP 酶; GATOR1 的缺失会使 mTORC1 信号对营养饥饿不敏感。 GATOR1成分与其他蛋白质没有序列同源性,因此GATOR1在分子水平上的功能目前尚不清楚。在这里,我们使用冷冻电子显微镜来解析 GATOR1 和 GATOR1-Rag GTPases 复合物的结构。 GATOR1采用中间空腔的扩展架构; NPRL2 连接 DEPDC5 和 NPRL3,DEPDC5 接触 Rag GTPase 异二聚体。生化分析表明,我们的 GATOR1-Rag GTPases 结构具有抑制性,并且 Rag GTPases 和 GATOR1 之间必须存在至少两种结合模式。 DEPDC5 与 RAGA 的直接相互作用抑制 GATOR1 介导的 RAGA 对 GTP 水解的刺激,而 NPRL2-NPRL3 异二聚体和 RAGA 之间较弱的相互作用执行 GAP 活性。这些数据揭示了营养感应 mTORC1 通路的一个组成部分的结构以及 GAP 与其底物 GTPase 之间的非典型相互作用。
更新日期:2018-03-28
中文翻译:

人类 GATOR1 和 GATOR1-Rag GTPases 复合物的结构
氨基酸和葡萄糖等营养物质通过 Rag GTPases 发出信号来激活 mTORC1。 GATOR1 蛋白复合物(包含 DEPDC5、NPRL2 和 NPRL3)作为 RAGA 的 GTP 酶激活蛋白 (GAP) 调节 Rag GTP 酶; GATOR1 的缺失会使 mTORC1 信号对营养饥饿不敏感。 GATOR1成分与其他蛋白质没有序列同源性,因此GATOR1在分子水平上的功能目前尚不清楚。在这里,我们使用冷冻电子显微镜来解析 GATOR1 和 GATOR1-Rag GTPases 复合物的结构。 GATOR1采用中间空腔的扩展架构; NPRL2 连接 DEPDC5 和 NPRL3,DEPDC5 接触 Rag GTPase 异二聚体。生化分析表明,我们的 GATOR1-Rag GTPases 结构具有抑制性,并且 Rag GTPases 和 GATOR1 之间必须存在至少两种结合模式。 DEPDC5 与 RAGA 的直接相互作用抑制 GATOR1 介导的 RAGA 对 GTP 水解的刺激,而 NPRL2-NPRL3 异二聚体和 RAGA 之间较弱的相互作用执行 GAP 活性。这些数据揭示了营养感应 mTORC1 通路的一个组成部分的结构以及 GAP 与其底物 GTPase 之间的非典型相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号