当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Numerical simulation of equilibrium ionic transport processes through permeable ion-exchange membranes in bi-ionic systems
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jelechem.2018.03.049 A.A. Moya
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jelechem.2018.03.049 A.A. Moya
|
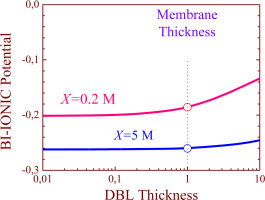
Abstract This paper aims to numerically study the equilibrium characteristics of the ionic inter-diffusion processes in ion-exchange membrane systems under bi-ionic conditions as a function of the membrane fixed-charge concentration and the thickness of the diffusion boundary layers adjacent to the membrane. The considered particular system is constituted by a membrane with negative fixed charge and two identical diffusion boundary layers on both sides of the membrane. The system is bathed by two solutions with a common co-ion but different counter-ion. The ionic transport processes of a ternary electrolyte are described by the Nernst-Planck-Poisson equations not only in the membrane but also in the diffusion layers, including the electric double layers at the interfaces. The ionic concentrations and electric potential profiles have been numerically obtained using the network simulation method in a permeable membrane system where the co-ion flux is a significant fraction of the counter-ionic fluxes. The effect of the diffusion boundary layers on the equilibrium membrane potential, the ionic fluxes and the ionic concentration profiles inside the membrane have been analysed and discussed.
更新日期:2018-05-01











































 京公网安备 11010802027423号
京公网安备 11010802027423号