当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Changes in Protein Dynamics in Escherichia coli SufS Reveal a Possible Conserved Regulatory Mechanism in Type II Cysteine Desulfurase Systems
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1021/acs.biochem.7b01275 Dokyong Kim 1 , Harsimran Singh 1 , Yuyuan Dai 2 , Guangchao Dong 2 , Laura S. Busenlehner 1 , F. Wayne Outten 2 , Patrick A. Frantom 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1021/acs.biochem.7b01275 Dokyong Kim 1 , Harsimran Singh 1 , Yuyuan Dai 2 , Guangchao Dong 2 , Laura S. Busenlehner 1 , F. Wayne Outten 2 , Patrick A. Frantom 1
Affiliation
|
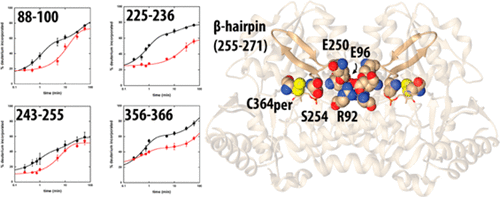
In the Suf Fe–S cluster assembly pathway, the activity of the cysteine desulfurase, SufS, is regulated by interactions with the accessory sulfotransferase protein, SufE. SufE has been shown to stimulate SufS activity, likely by inducing conformational changes in the SufS active site that promote the desulfurase step and by acting as an efficient persulfide acceptor in the transpersulfuration step. Previous results point toward an additional level of regulation through a “half-sites” mechanism that affects the stoichiometry and affinity for SufE as the dimeric SufS shifts between desulfurase and transpersulfuration activities. Investigation of the covalent persulfide intermediate of SufS by backbone amide hydrogen–deuterium exchange mass spectrometry identified two active site peptides (residues 225–236 and 356–366) and two peptides at the dimer interface of SufS (residues 88–100 and 243–255) that exhibit changes in deuterium uptake upon formation of the intermediate. Residues in these peptides are organized to form a conduit between the two active sites upon persulfide formation and include key cross-monomer interactions, suggesting they may play a role in the half-sites regulation. Three evolutionarily conserved residues at the dimer interface (R92, E96, and E250) were investigated by alanine scanning mutagenesis. Two of the substituted enzymes (E96A and E250A SufS) resulted in 6-fold increases in the value of KSufE, confirming a functional role. Re-examination of the dimer interface in reported crystal structures of SufS and the SufS homologue CsdA identified previously unnoticed residue mobility at the dimer interface. The identification of conformational changes at the dimer interface by hydrogen–deuterium exchange confirmed by mutagenesis and structural reports provides a physical mechanism for active site communication in the half-sites regulation of SufS activity. Given the conservation of the interface interactions, this mechanism may be broadly applicable to type II cysteine desulfurase systems.
中文翻译:

大肠杆菌SufS中蛋白质动力学的变化揭示了II型半胱氨酸脱硫酶系统中可能的保守调控机制。
在Suf Fe–S簇组装途径中,半胱氨酸脱硫酶SufS的活性受与辅助硫转移酶蛋白SufE相互作用的调节。已显示SufE可以刺激SufS活性,可能是通过在SufS活性位点中诱导构象变化来促进脱硫酶步骤,并在反全硫步骤中充当有效的过硫受体。先前的研究结果表明,通过二聚体SufS在脱硫酶和反全硫反应之间转移,会通过“半位点”机制提高调节水平,这种机制会影响化学计量和对SufE的亲和力。通过骨架酰胺氢-氘交换质谱法对SufS的共价过硫化中间体进行的研究确定了两个活性位点肽(残基225-236和356-366)和在SufS的二聚体界面处的两个肽(残基88-100和243-255) )在形成中间体时氘的吸收发生变化。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并且包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。K SufE,确认其功能作用。在报告的SufS和SufS同源CsdA晶体结构中对二聚体界面的重新检查确定了以前在二聚体界面处未被注意到的残基迁移率。通过诱变和结构报告证实通过氢-氘交换在二聚体界面处构象变化的鉴定为SufS活性的半位点调节中的活性位点通讯提供了物理机制。考虑到界面相互作用的保守性,该机制可广泛应用于II型半胱氨酸脱硫酶系统。
更新日期:2018-03-28
中文翻译:

大肠杆菌SufS中蛋白质动力学的变化揭示了II型半胱氨酸脱硫酶系统中可能的保守调控机制。
在Suf Fe–S簇组装途径中,半胱氨酸脱硫酶SufS的活性受与辅助硫转移酶蛋白SufE相互作用的调节。已显示SufE可以刺激SufS活性,可能是通过在SufS活性位点中诱导构象变化来促进脱硫酶步骤,并在反全硫步骤中充当有效的过硫受体。先前的研究结果表明,通过二聚体SufS在脱硫酶和反全硫反应之间转移,会通过“半位点”机制提高调节水平,这种机制会影响化学计量和对SufE的亲和力。通过骨架酰胺氢-氘交换质谱法对SufS的共价过硫化中间体进行的研究确定了两个活性位点肽(残基225-236和356-366)和在SufS的二聚体界面处的两个肽(残基88-100和243-255) )在形成中间体时氘的吸收发生变化。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并且包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。这些肽中的残基在过硫化物形成时被组织成在两个活性位点之间形成导管,并包括关键的交叉单体相互作用,表明它们可能在半位点调节中发挥作用。通过丙氨酸扫描诱变研究了二聚体界面上的三个进化保守残基(R92,E96和E250)。两种被取代的酶(E96A和E250A SufS)使的值增加了6倍。K SufE,确认其功能作用。在报告的SufS和SufS同源CsdA晶体结构中对二聚体界面的重新检查确定了以前在二聚体界面处未被注意到的残基迁移率。通过诱变和结构报告证实通过氢-氘交换在二聚体界面处构象变化的鉴定为SufS活性的半位点调节中的活性位点通讯提供了物理机制。考虑到界面相互作用的保守性,该机制可广泛应用于II型半胱氨酸脱硫酶系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号