当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Subnanometer Gold Clusters Adhere to Lipid A for Protection against Endotoxin-Induced Sepsis
Nano Letters ( IF 9.6 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1021/acs.nanolett.7b05464 Fang-Hsuean Liao,Te-Haw Wu,Yu-Ting Huang,Wen-Jye Lin,Chun-Jen Su,U-Ser Jeng,Shu-Chen Kuo,Shu-Yi Lin
Nano Letters ( IF 9.6 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1021/acs.nanolett.7b05464 Fang-Hsuean Liao,Te-Haw Wu,Yu-Ting Huang,Wen-Jye Lin,Chun-Jen Su,U-Ser Jeng,Shu-Chen Kuo,Shu-Yi Lin
|
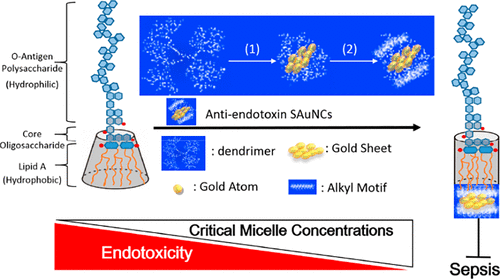
Endotoxicity originating from a dangerous debris (i.e., lipopolysaccharide, LPS) of Gram-negative bacteria is a challenging clinical problem, but no drugs or therapeutic strategies that can successfully address this issue have been identified yet. In this study, we report a subnanometer gold cluster that can efficiently block endotoxin activity to protect against sepsis. The endotoxin blocker consists of a gold nanocluster that serves as a flakelike substrate and a coating of short alkyl motifs that act as an adhesive to dock with LPS by compacting the intramolecular hydrocarbon chain–chain distance (d-spacing) of lipid A, an endotoxicity active site that can cause overwhelming cytokine induction resulting in sepsis progression. Direct evidence showed the d-spacing values of lipid A to be decreased from 4.19 Å to either 3.85 or 3.54 Å, indicating more dense packing densities in the presence of subnanometer gold clusters. In terms of biological relevance, the concentrations of key pro-inflammatory NF-κB-dependent cytokines, including plasma TNF-α, IL-6, and IL-1β, and CXC chemokines, in LPS-challenged mice showed a noticeable decrease. More importantly, we demonstrated that the treatment of antiendotoxin gold nanoclusters significantly prolonged the survival time in LPS-induced septic mice. The ultrasmall gold nanoclusters could target lipid A of LPS to deactivate endotoxicity by compacting its packing density, which might constitute a potential therapeutic strategy for the early prevention of sepsis caused by Gram-negative bacterial infection.
中文翻译:

亚纳米级金团簇附着在脂质A上,可预防内毒素引起的脓毒症
革兰氏阴性细菌的危险碎片(即脂多糖,LPS)引起的内毒性是一个具有挑战性的临床问题,但是尚未找到能够成功解决该问题的药物或治疗策略。在这项研究中,我们报告了一个亚纳米级的金簇,它可以有效地阻止内毒素的活性,以预防败血症。内毒素阻滞剂由充当薄片状底物的金纳米簇和短烷基基序涂层组成,这些涂层通过压实脂质A的分子内烃链-链距(d间距)而与LPS对接,这是一种内毒素可能导致压倒性细胞因子诱导的活性位点,导致败血症进展。直接的证据表明,d脂质A的-spacing值将从4.19Å降低到3.85或3.54Å,表明在存在亚纳米金簇的情况下堆积密度更高。在生物学相关性方面,LPS攻击的小鼠中关键促炎性NF-κB依赖性细胞因子(包括血浆TNF-α,IL-6和IL-1β和CXC趋化因子)的浓度显着降低。更重要的是,我们证明了抗内毒素金纳米簇的治疗显着延长了LPS诱导的败血症小鼠的存活时间。超小型金纳米簇可以通过压缩脂多糖的堆积密度来靶向脂多糖脂类,从而使内毒素失活,这可能是早期预防革兰氏阴性细菌感染引起败血症的潜在治疗策略。
更新日期:2018-03-28
中文翻译:

亚纳米级金团簇附着在脂质A上,可预防内毒素引起的脓毒症
革兰氏阴性细菌的危险碎片(即脂多糖,LPS)引起的内毒性是一个具有挑战性的临床问题,但是尚未找到能够成功解决该问题的药物或治疗策略。在这项研究中,我们报告了一个亚纳米级的金簇,它可以有效地阻止内毒素的活性,以预防败血症。内毒素阻滞剂由充当薄片状底物的金纳米簇和短烷基基序涂层组成,这些涂层通过压实脂质A的分子内烃链-链距(d间距)而与LPS对接,这是一种内毒素可能导致压倒性细胞因子诱导的活性位点,导致败血症进展。直接的证据表明,d脂质A的-spacing值将从4.19Å降低到3.85或3.54Å,表明在存在亚纳米金簇的情况下堆积密度更高。在生物学相关性方面,LPS攻击的小鼠中关键促炎性NF-κB依赖性细胞因子(包括血浆TNF-α,IL-6和IL-1β和CXC趋化因子)的浓度显着降低。更重要的是,我们证明了抗内毒素金纳米簇的治疗显着延长了LPS诱导的败血症小鼠的存活时间。超小型金纳米簇可以通过压缩脂多糖的堆积密度来靶向脂多糖脂类,从而使内毒素失活,这可能是早期预防革兰氏阴性细菌感染引起败血症的潜在治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号