当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Thermodynamic Model for Redox-Dependent Binding of Carbon Monoxide at Site-Differentiated, High Spin Iron Clusters
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-28 , DOI: 10.1021/jacs.8b01825 Charles H Arnett 1 , Matthew J Chalkley 1 , Theodor Agapie 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-28 , DOI: 10.1021/jacs.8b01825 Charles H Arnett 1 , Matthew J Chalkley 1 , Theodor Agapie 1
Affiliation
|
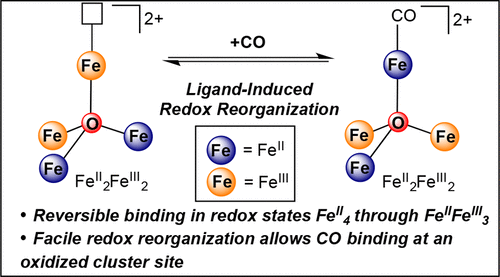
Binding of N2 and CO by the FeMo-cofactor of nitrogenase depends on the redox level of the cluster, but the extent to which pure redox chemistry perturbs the affinity of high spin iron clusters for π-acids is not well understood. Here, we report a series of site-differentiated iron clusters that reversibly bind CO in redox states FeII4 through FeIIFeIII3. One electron redox events result in small changes in the affinity for (at most ∼400-fold) and activation of CO (at most 28 cm-1 for νCO). The small influence of redox chemistry on the affinity of these high spin, valence-localized clusters for CO is in stark contrast to the large enhancements (105-1022 fold) in π-acid affinity reported for monometallic and low spin, bimetallic iron complexes, where redox chemistry occurs exclusively at the ligand binding site. While electron-loading at metal centers remote from the substrate binding site has minimal influence on the CO binding energetics (∼1 kcal·mol-1), it provides a conduit for CO binding at an FeIII center. Indeed, internal electron transfer from these remote sites accommodates binding of CO at an FeIII, with a small energetic penalty arising from redox reorganization (∼2.6 kcal·mol-1). The ease with which these clusters redistribute electrons in response to ligand binding highlights a potential pathway for coordination of N2 and CO by FeMoco, which may occur on an oxidized edge of the cofactor.
中文翻译:

一氧化碳在位点分化的高自旋铁簇上的氧化还原依赖性结合的热力学模型
固氮酶的 FeMo 辅因子对 N2 和 CO 的结合取决于簇的氧化还原水平,但纯氧化还原化学扰乱高自旋铁簇对 π 酸的亲和力的程度尚不清楚。在这里,我们报道了一系列位点分化的铁簇,它们以氧化还原态 FeII4 到 FeIIFeIII3 可逆地结合 CO。一个电子氧化还原事件会导致 CO 的亲和力(最多 ∼400 倍)和活化(νCO 最多 28 cm-1)发生微小变化。氧化还原化学对这些高自旋、价态局域簇对 CO 的亲和力的微小影响与报道的单金属和低自旋双金属铁络合物的 π 酸亲和力的大幅增强(105-1022 倍)形成鲜明对比。其中氧化还原化学仅发生在配体结合位点。虽然远离底物结合位点的金属中心的电子负载对 CO 结合能量 (∼1 kcal·mol-1) 的影响很小,但它为 FeIII 中心的 CO 结合提供了通道。事实上,来自这些远程位点的内部电子转移适应了 CO 在 FeIII 上的结合,并因氧化还原重组而产生了小的能量损失 (∼2.6 kcal·mol-1)。这些簇响应配体结合而轻松地重新分配电子,凸显了 FeMoco 协调 N2 和 CO 的潜在途径,这可能发生在辅因子的氧化边缘上。
更新日期:2018-03-28
中文翻译:

一氧化碳在位点分化的高自旋铁簇上的氧化还原依赖性结合的热力学模型
固氮酶的 FeMo 辅因子对 N2 和 CO 的结合取决于簇的氧化还原水平,但纯氧化还原化学扰乱高自旋铁簇对 π 酸的亲和力的程度尚不清楚。在这里,我们报道了一系列位点分化的铁簇,它们以氧化还原态 FeII4 到 FeIIFeIII3 可逆地结合 CO。一个电子氧化还原事件会导致 CO 的亲和力(最多 ∼400 倍)和活化(νCO 最多 28 cm-1)发生微小变化。氧化还原化学对这些高自旋、价态局域簇对 CO 的亲和力的微小影响与报道的单金属和低自旋双金属铁络合物的 π 酸亲和力的大幅增强(105-1022 倍)形成鲜明对比。其中氧化还原化学仅发生在配体结合位点。虽然远离底物结合位点的金属中心的电子负载对 CO 结合能量 (∼1 kcal·mol-1) 的影响很小,但它为 FeIII 中心的 CO 结合提供了通道。事实上,来自这些远程位点的内部电子转移适应了 CO 在 FeIII 上的结合,并因氧化还原重组而产生了小的能量损失 (∼2.6 kcal·mol-1)。这些簇响应配体结合而轻松地重新分配电子,凸显了 FeMoco 协调 N2 和 CO 的潜在途径,这可能发生在辅因子的氧化边缘上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号