当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the alternatives of biological nitrogen fixation
Metallomics ( IF 2.9 ) Pub Date : 2018-03-28 , DOI: 10.1039/c8mt00038g Florence Mus 1, 2, 3 , Alexander B. Alleman 1, 2, 3 , Natasha Pence 3, 4, 5 , Lance C. Seefeldt 3, 6, 7 , John W. Peters 1, 2, 3
Metallomics ( IF 2.9 ) Pub Date : 2018-03-28 , DOI: 10.1039/c8mt00038g Florence Mus 1, 2, 3 , Alexander B. Alleman 1, 2, 3 , Natasha Pence 3, 4, 5 , Lance C. Seefeldt 3, 6, 7 , John W. Peters 1, 2, 3
Affiliation
|
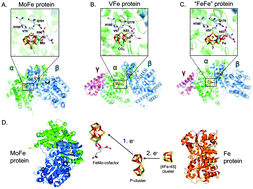
Most biological nitrogen fixation (BNF) results from the activity of the molybdenum nitrogenase (Mo-nitrogenase, Nif), an oxygen-sensitive metalloenzyme complex found in all known diazotrophs. Two alternative forms of nitrogenase, the vanadium nitrogenase (V-nitrogenase, Vnf) and the iron-only nitrogenase (Fe-only nitrogenase, Anf) have also been identified in the genome of some organisms that encode for Nif. It has been suggested that alternative nitrogenases were responsible for N2-fixation on early Earth because oceans were depleted of bioavailable Mo. Results of recent phylogenetic- and structure-based studies suggest, however, that such an evolutionary path is unlikely, and favor a new model for a stepwise evolution of nitrogenase where the V-nitrogenase and the Fe-only nitrogenase are not the ancestor of the Mo-nitrogenase. Rather, Mo-nitrogenase emerged within the methanogenic archaea and then gave rise to the alternative forms suggesting they arose later in response to the availability of fixed N2 and local environmental factors that influenced metal availability. This review summarizes the current state of knowledge on (1) the biochemistry of these complex systems highlighting the common and specific structural features and catalytic activities of the enzymes, (2) the recent progress in defining the discrete set of genes associated to N2-fixation and the regulatory features that coordinate the differential expression of genes in response to metal availability, and (3) the diverse taxonomic and phylogenic distribution of nitrogenase enzymes and the evolutionary history of BNF from the perspective of metal content and metal availability.
中文翻译:

探索生物固氮的替代方法
大多数生物固氮作用(BNF)是由钼固氮酶(Mo-nitrogenase,Nif)的活性产生的,钼固氮酶是一种在所有已知的重氮营养物中都发现的对氧敏感的金属酶复合物。在编码Nif的某些生物的基因组中,还发现了两种其他形式的固氮酶:钒固氮酶(V-固氮酶,Vnf)和仅固铁固氮酶(仅固铁固氮酶,Anf)。有人提出,替代的固氮酶是造成N 2的原因。固定在地球早期,因为海洋中已耗尽了生物可利用的Mo。然而,最近基于系统发育和结构的研究结果表明,这种进化路径不太可能,并且倾向于采用新的模型来逐步分解固氮酶,其中V-固氮酶和仅含铁的固氮酶不是钼固氮酶的祖先。相反,Mo-硝化酶出现在产甲烷的古细菌中,然后产生了其他形式,表明它们是后来随着固定N 2的出现而出现的。以及影响金属可用性的当地环境因素。本文总结(1)上的,这些复杂体系突出了公共和特定的结构特征和酶的催化活性的生物化学知识的当前状态,(2)在确定相关联的N个离散组基因的最新进展2 -固定和调节功能,协调基因的差异表达以响应金属的可利用性,以及(3)从金属含量和金属可利用性的角度来看,固氮酶的分类和系统发育的多样性以及BNF的进化史。
更新日期:2018-04-25
中文翻译:

探索生物固氮的替代方法
大多数生物固氮作用(BNF)是由钼固氮酶(Mo-nitrogenase,Nif)的活性产生的,钼固氮酶是一种在所有已知的重氮营养物中都发现的对氧敏感的金属酶复合物。在编码Nif的某些生物的基因组中,还发现了两种其他形式的固氮酶:钒固氮酶(V-固氮酶,Vnf)和仅固铁固氮酶(仅固铁固氮酶,Anf)。有人提出,替代的固氮酶是造成N 2的原因。固定在地球早期,因为海洋中已耗尽了生物可利用的Mo。然而,最近基于系统发育和结构的研究结果表明,这种进化路径不太可能,并且倾向于采用新的模型来逐步分解固氮酶,其中V-固氮酶和仅含铁的固氮酶不是钼固氮酶的祖先。相反,Mo-硝化酶出现在产甲烷的古细菌中,然后产生了其他形式,表明它们是后来随着固定N 2的出现而出现的。以及影响金属可用性的当地环境因素。本文总结(1)上的,这些复杂体系突出了公共和特定的结构特征和酶的催化活性的生物化学知识的当前状态,(2)在确定相关联的N个离散组基因的最新进展2 -固定和调节功能,协调基因的差异表达以响应金属的可利用性,以及(3)从金属含量和金属可利用性的角度来看,固氮酶的分类和系统发育的多样性以及BNF的进化史。











































 京公网安备 11010802027423号
京公网安备 11010802027423号