Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-28 , DOI: 10.1016/j.tetlet.2018.03.081 Vijai K. Rai , Fooleswar Verma , Mansingh Satnami , Manorama Singh , Ankita Rai
|
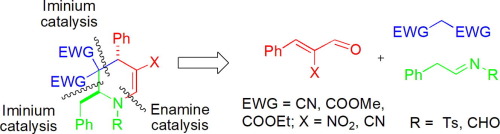
A novel and efficient three-component coupling strategy for stereoselective synthesis of highly substituted tetrahydropyridines (THP) is reported in high yield (83–94%) with excellent diastereoselectivity (95–99%) in favor of anti-isomer. The reaction proceeds via sequential iminium-iminium-enamine mediated formation of three consecutive CC, C
C and C
N bonds in one-pot through reaction of [E]-α-cyano/nitro unsaturated aldehyde, activated methylene and aldimine/phenyl-N-tosyl-methanimine and opens up a new aspect for the utility of Morita-Baylis-Hillman (MBH) adducts in THP synthesis.
中文翻译:

基于Morita-Baylis-Hillman烯类的三级联反应策略,用于使用亚胺-烯胺催化反选择性合成高度官能化的四氢吡啶
据报道,一种新颖且高效的三组分偶联策略可实现高取代度的四氢吡啶(THP)的立体选择性合成,产率高(83-94%),非对映选择性(95-99%),有利于抗异构体。该反应通过[ E ]-α-氰基/硝基不饱和醛,活化的亚甲基和醛亚胺/苯基-的反应,在一个锅中依次由亚胺-亚胺-亚胺介导,依次形成三个连续的C C,C
C和C
N键。N-甲苯磺酰基-亚甲基胺为Morita-Baylis-Hillman(MBH)加合物在THP合成中的应用开辟了一个新的领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号