当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Storage Time Improve the Physical Stability of Amorphous Pharmaceuticals with Tautomerization Ability Exposed to Compression? The Case of a Chloramphenicol Drug
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00099 Justyna Knapik-Kowalczuk 1, 2 , Zaneta Wojnarowska 1, 2, 3 , Krzysztof Chmiel 1, 2 , Marzena Rams-Baron 1, 2 , Lidia Tajber 3 , Marian Paluch 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00099 Justyna Knapik-Kowalczuk 1, 2 , Zaneta Wojnarowska 1, 2, 3 , Krzysztof Chmiel 1, 2 , Marzena Rams-Baron 1, 2 , Lidia Tajber 3 , Marian Paluch 1, 2
Affiliation
|
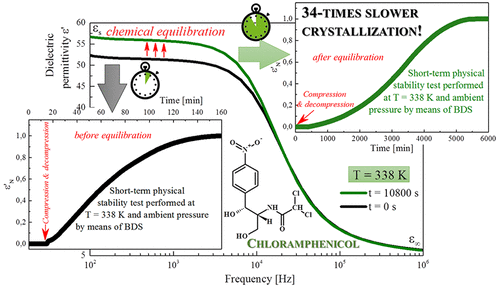
In this article we thoroughly investigated the physical stability of the amorphous form of a chloramphenicol drug. The tendency toward recrystallization of this drug has been examined (i) at nonisothermal conditions by means of a DSC technique; (ii) at isothermal conditions and temperature close to Troom by means of dielectric spectroscopy; (iii) at isothermal conditions and elevated temperatures of T = 323 K and 338 K by dielectric spectroscopy; and (iv) at conditions imitating the manufacturing procedure (i.e., elevated temperature and compression procedure). Our investigations have shown that amorphous chloramphenicol, stored at both standard storage and elevated temperature conditions, does not reveal a tendency toward recrystallization. However, compression significantly changes this behavior and destabilizes the examined compound. We found that due to chemical equilibration of the sample, the elongation of the storage time before compression might improve the physical stability of the examined pharmaceutical exposed to compression 34-times.
中文翻译:

储存时间是否可以通过压缩使互变异构能力提高无定形药物的物理稳定性?氯霉素药物的情况
在本文中,我们彻底研究了氯霉素药物无定形形式的物理稳定性。(i)在非等温条件下通过DSC技术检查了该药物的重结晶趋势;(ii)在等温条件下,通过介电光谱法接近T室;(iii)在等温条件和T升高的温度下介电光谱法测得= 323K和338K;(iv)在模仿制造程序的条件下(即高温和压缩程序)。我们的研究表明,在标准储存条件和高温条件下储存的无定形氯霉素均未显示出重结晶的趋势。但是,压缩会显着改变此行为,并使所检查的化合物不稳定。我们发现由于样品的化学平衡,压缩前存储时间的延长可能会改善被压缩34次的受检药物的物理稳定性。
更新日期:2018-03-27
中文翻译:

储存时间是否可以通过压缩使互变异构能力提高无定形药物的物理稳定性?氯霉素药物的情况
在本文中,我们彻底研究了氯霉素药物无定形形式的物理稳定性。(i)在非等温条件下通过DSC技术检查了该药物的重结晶趋势;(ii)在等温条件下,通过介电光谱法接近T室;(iii)在等温条件和T升高的温度下介电光谱法测得= 323K和338K;(iv)在模仿制造程序的条件下(即高温和压缩程序)。我们的研究表明,在标准储存条件和高温条件下储存的无定形氯霉素均未显示出重结晶的趋势。但是,压缩会显着改变此行为,并使所检查的化合物不稳定。我们发现由于样品的化学平衡,压缩前存储时间的延长可能会改善被压缩34次的受检药物的物理稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号