当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor Pressure and Heat of Vaporization of Molecules That Associate in the Gas Phase
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-16 , DOI: 10.1021/acs.iecr.7b04241 Radomir I. Slavchov 1 , Javor K. Novev 2 , Sebastian Mosbach 1 , Markus Kraft 1, 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-16 , DOI: 10.1021/acs.iecr.7b04241 Radomir I. Slavchov 1 , Javor K. Novev 2 , Sebastian Mosbach 1 , Markus Kraft 1, 3
Affiliation
|
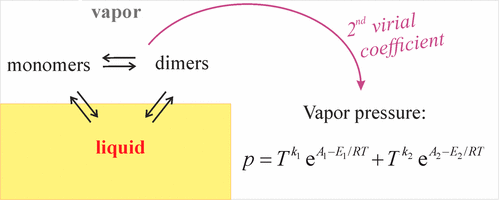
A model of the temperature dependence of the vapor pressure and the heat of vaporization of liquids whose vapors contain associates is presented for two cases: dimers and linear associates in the gas phase. The results are analytic generalizations of the Clausius–Clapeyron equation, valid with accuracy of 0.1%–1%, as demonstrated with 11 liquids: formic and acetic acids, methanol, ethanol, n-propanol, n-butanol, water, benzene, toluene, heptane, and isooctane. The model involves only readily available handbook parameters: the room-temperature heat of vaporization, vapor pressure, heat capacities, second virial coefficient, and heat of dissociation of the dimers in the gas phase.
中文翻译:

气相中的分子的蒸气压和蒸气化热
针对两种情况,提出了一种蒸汽压力与温度的关系模型,该模型中的蒸汽中含有缔合体的液体的汽化热为两种情况:气相中的二聚体和线性缔合体。结果是Clausius–Clapeyron方程的解析概括,精度为0.1%–1%,适用于11种液体:甲酸和乙酸,甲醇,乙醇,正丙醇,正丁醇,水,苯,甲苯,庚烷和异辛烷。该模型仅涉及易于获得的手册参数:室温下的汽化热,蒸汽压,热容量,第二维里系数和气相中二聚体的解离热。
更新日期:2018-04-16
中文翻译:

气相中的分子的蒸气压和蒸气化热
针对两种情况,提出了一种蒸汽压力与温度的关系模型,该模型中的蒸汽中含有缔合体的液体的汽化热为两种情况:气相中的二聚体和线性缔合体。结果是Clausius–Clapeyron方程的解析概括,精度为0.1%–1%,适用于11种液体:甲酸和乙酸,甲醇,乙醇,正丙醇,正丁醇,水,苯,甲苯,庚烷和异辛烷。该模型仅涉及易于获得的手册参数:室温下的汽化热,蒸汽压,热容量,第二维里系数和气相中二聚体的解离热。











































 京公网安备 11010802027423号
京公网安备 11010802027423号