当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective conjugate addition/cyclization/bromination: Access to four stereocenters in a single step
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.tetlet.2018.03.080 Junzhuo Liao , Dale G. Drueckhammer
中文翻译:

非对映选择性共轭加成/环化/溴化:一步即可进入四个立体中心
更新日期:2018-03-27
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.tetlet.2018.03.080 Junzhuo Liao , Dale G. Drueckhammer
|
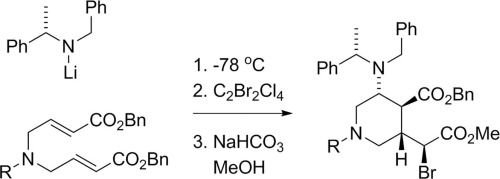
A stereoselective tandem conjugate addition reaction with a chiral amine-derived nucleophile is reported in which the enolate intermediate is quenched with 1,2-dibromotetrachloroethane as a mild brominating reagent. X-ray analysis of a subsequent derivative was used to prove the configuration at each of the four newly formed stereocenters. The resulting α-bromoester underwent selective transesterification catalyzed by mild base to allow selective manipulation of the two ester groups of the product.
中文翻译:

非对映选择性共轭加成/环化/溴化:一步即可进入四个立体中心
报道了与手性胺衍生的亲核试剂的立体选择性串联共轭加成反应,其中烯醇酸酯中间体用1,2-二溴四氯乙烷作为温和的溴化试剂淬灭。随后的导数的X射线分析被用来证明在四个新形成的立体中心的每一个处的构型。所得的α-溴酸酯经过弱碱催化选择性酯交换,以允许产物的两个酯基的选择性操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号